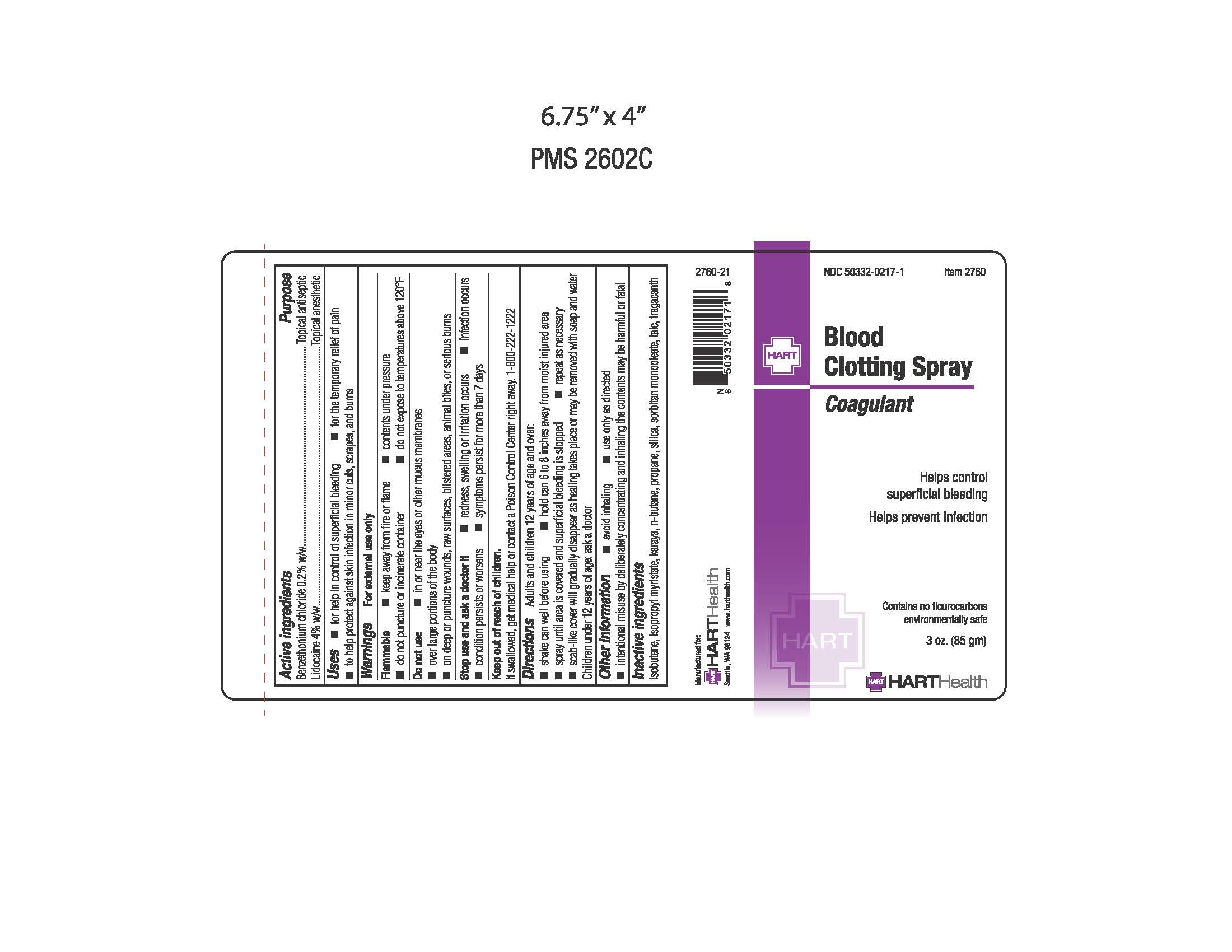
Label: BLOOD CLOTTING- benzethonium chloride and lidocaine aerosol, spray
- NDC Code(s): 50332-0217-1
- Packager: HART Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Do not use
- Stop use and ask doctor if
- Keep out of reach of children.
-
Directions
Adults and children 23 years of age and over:
- shake can well before using
- hold 6-8 inches from moist injured area
- spray until area is covered and superficial bleeding is stopped
- repeat as necessary
- scab-like cover will gradually disappear as healing takes place or may be removed with soap and water
Children under 12 years of age: ask a doctor
- Other Information
- Inactive Ingredients
- Principal Display
-
INGREDIENTS AND APPEARANCE
BLOOD CLOTTING
benzethonium chloride and lidocaine aerosol, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50332-0217 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 3400 mg in 85 g BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 170 mg in 85 g Inactive Ingredients Ingredient Name Strength ISOBUTANE (UNII: BXR49TP611) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) KARAYA GUM (UNII: 73W9IQY50Q) BUTANE (UNII: 6LV4FOR43R) PROPANE (UNII: T75W9911L6) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 3-(TRIETHOXYSILYL)PROPYLAMINE (UNII: L8S6UBW552) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) TALC (UNII: 7SEV7J4R1U) TRAGACANTH (UNII: 2944357O2O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50332-0217-1 85 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 04/01/1974 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 04/01/1974 Labeler - HART Health (069560969)