Label: AT ONE WITH NATURE- menthol 1% gel, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 54602-011-05 - Packager: BIOCARE LABS INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 3, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
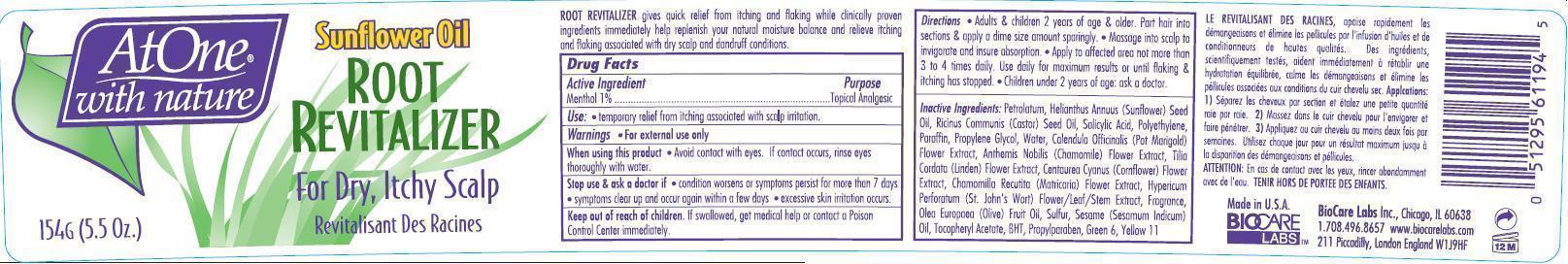
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
-
Directions
- Adult & children 2 year of age & older. Part hair into section & apply a dime size amount sparingly.
- Massage into scalp to invigorate and insure absorption.
- Apply to affected area not more then 3 to 4 times daily. Use daily for maximum results or flaking & itching has stopped.
- Children under 2 years of age ask a doctor
-
INACTIVE INGREDIENTS
Petrolatum, Helianthus Annuus (Sunflower) Seed Oil, Ricinus Communis (Castor) Seed Oil, Salicylic Acid, Polyethylene, Paraffin, Propylene Glycol, Water, Calendula Officinalis (Pot Marigold ) Flower Extract, Anthemis Nobilis Chamomile Flower Extract , Tilia Cordata (Linden)Flower Extract, Centaurea Cyanus (Cornflower) Flower Extract, Chamomilla Recutita (Matricaria) Flower Extract, Hypericum Perforatum Extract (St. John's Wort) Extract, Fragrance, Olea Europaea (Olive) Fruit Oil, Sulfur, Sesame (Sesamum Indicum) Oil, Tocopheryl Acetate, BHT, Propylparaben, Green 6, Yellow 11
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AT ONE WITH NATURE
menthol 1% gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54602-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Menthol (UNII: L7T10EIP3A) (Menthol - UNII:L7T10EIP3A) Menthol 10 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) SUNFLOWER OIL (UNII: 3W1JG795YI) CASTOR OIL (UNII: D5340Y2I9G) SALICYLIC ACID (UNII: O414PZ4LPZ) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) PARAFFIN (UNII: I9O0E3H2ZE) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) TILIA CORDATA FLOWER (UNII: CFN6G1F6YK) CENTAUREA CYANUS FLOWER (UNII: QZ239038YC) CHAMOMILE (UNII: FGL3685T2X) HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) OLIVE OIL (UNII: 6UYK2W1W1E) SULFUR (UNII: 70FD1KFU70) SESAME OIL (UNII: QX10HYY4QV) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PROPYLPARABEN (UNII: Z8IX2SC1OH) D&C GREEN NO. 6 (UNII: 4QP5U84YF7) D&C YELLOW NO. 11 (UNII: 44F3HYL954) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54602-011-05 154 g in 1 CANISTER 09/18/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/18/2014 Labeler - BIOCARE LABS INC. (798953493) Establishment Name Address ID/FEI Business Operations RNA PHARMA, LLC 079103999 manufacture(54602-011)