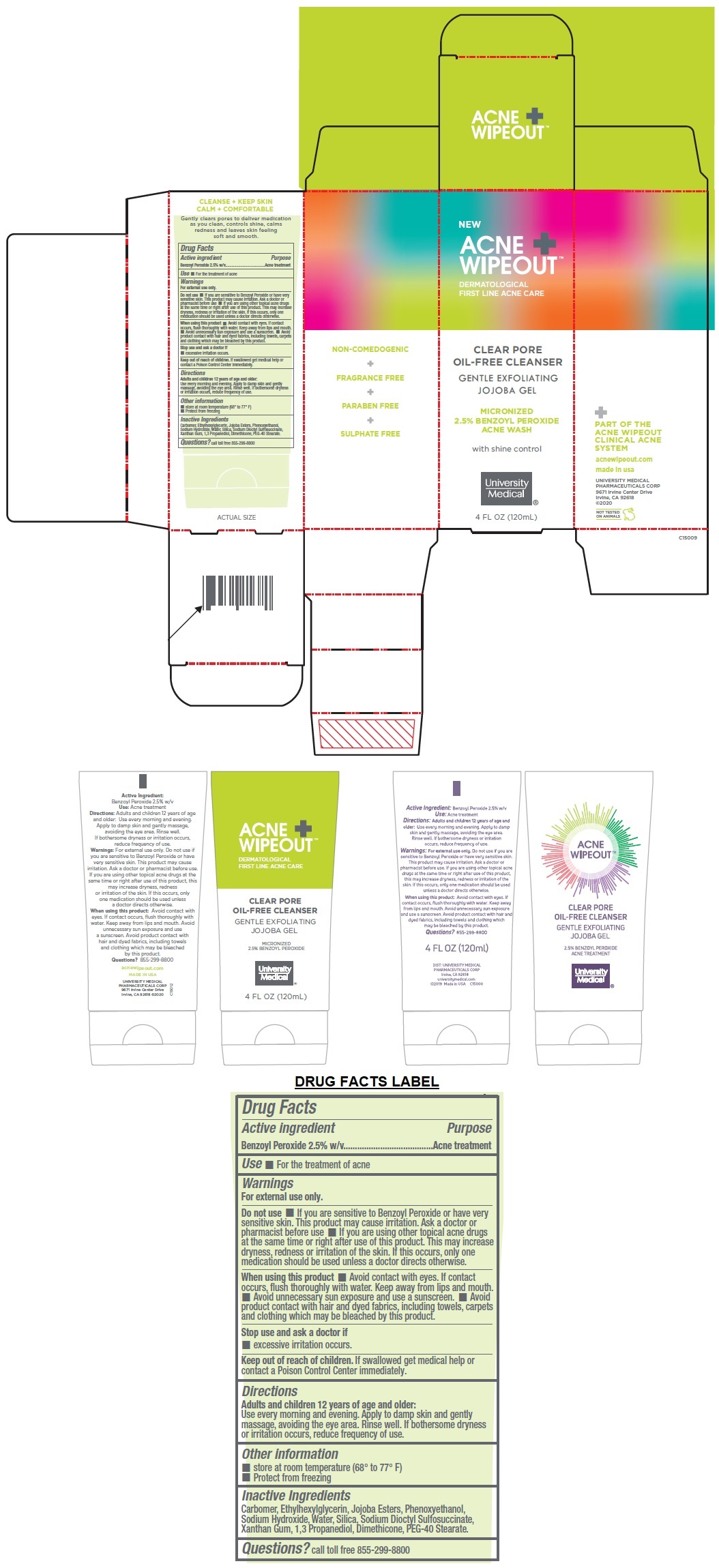
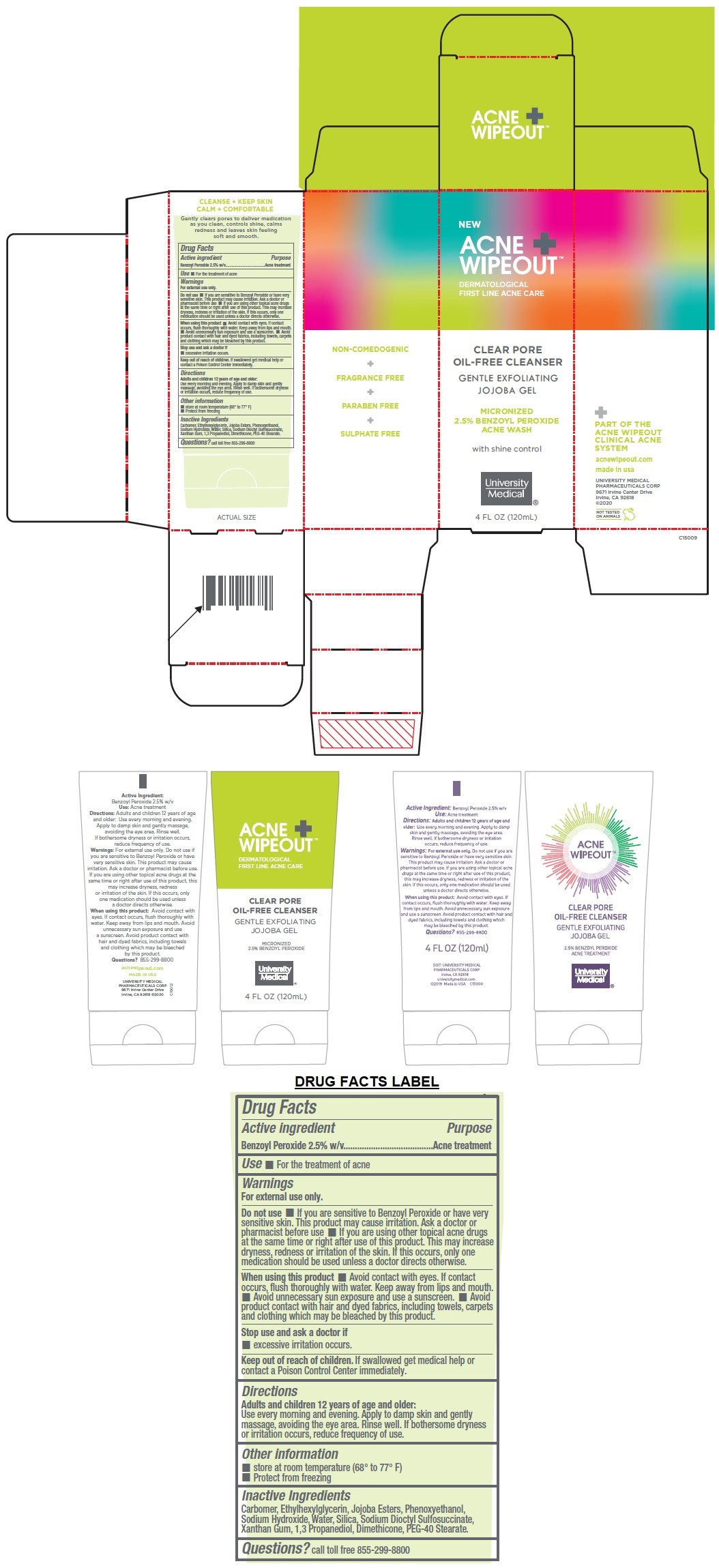
Label: ACNE WIPEOUT CLEAR PORE OIL-FREE CLEANSER- benzoyl peroxide gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 50544-151-00 - Packager: University Medical Pharmaceuticals Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 2, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only.
Do not use • If you are sensitive to Benzoyl Peroxide or have very sensitive skin. This product may cause irritation. Ask a doctor or pharmacist before use • If you are using other topical acne drugs at the same time or right after use of this product. This may increase dryness, redness or irritation of the skin. If this occurs, only one medication should be used unless a doctor directs otherwise.
When using this product • Avoid contact with eyes. If contact occurs, flush thoroughly with water. Keep away from lips and mouth. • Avoid unnecessary sun exposure and use a sunscreen. • Avoid product contact with hair and dyed fabrics, including towels, carpets and clothing which may be bleached by this product.
Stop use and ask a doctor if
• excessive irritation occurs. - Directions
- Other information
- Inactive Ingredients
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
DERMATOLOGICAL FIRST LINE ACNE CARE
GENTLE EXFOLIATING JOJOBA GEL
MICRONIZED 2.5% BENZOYL PEROXIDE ACNE WASH
with shine control
PART OF THE ACNE WIPEOUT CLINICAL ACNE SYSTEM
acnewipeout.com
made in usa
UNIVERSITY MEDICAL
PHARMACEUTICALS CORP
9671 Irvine Center Drive
Irvine, CA 92618
©2020
NOT TESTED ON ANIMALS
NON-COMEDOGENIC + FRAGRANCE FREE + PARABEN FREE + SULPHATE FREE
CLEANSE + KEEP SKIN
CALM + COMFORTABLE
Gently clears pores to deliver medication as you clean, controls shine, calms redness and leaves skin feeling soft and smooth.
- Packaging
-
INGREDIENTS AND APPEARANCE
ACNE WIPEOUT CLEAR PORE OIL-FREE CLEANSER
benzoyl peroxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50544-151 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 2.5 g in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) JOJOBA OIL (UNII: 724GKU717M) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DOCUSATE SODIUM (UNII: F05Q2T2JA0) XANTHAN GUM (UNII: TTV12P4NEE) PROPANEDIOL (UNII: 5965N8W85T) DIMETHICONE (UNII: 92RU3N3Y1O) PEG-40 STEARATE (UNII: ECU18C66Q7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50544-151-00 1 in 1 CARTON 06/01/2020 1 120 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 06/01/2020 Labeler - University Medical Pharmaceuticals Corp. (809706252)