Label: EXELDERM- sulconazole nitrate cream
- NDC Code(s): 69489-711-60
- Packager: Journey Medical Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 19, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
EXELDERM (sulconazole nitrate, USP) CREAM, 1.0% is a broad-spectrum antifungal agent intended for topical application. Sulconazole nitrate, USP, the active ingredient in EXELDERM CREAM, is an imidazole derivative with in vitro antifungal and antiyeast activity. Its chemical name is (±)-1-[2,4-dichloro-β-[(p-chlorobenzyl)-thiol]-phenethyl]imidazole mononitrate and it has the following chemical structure:
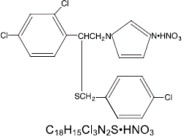
Sulconazole nitrate, USP is a white to off-white crystalline powder with a molecular weight of 460.77. It is freely soluble in pyridine; slightly soluble in ethanol, acetone, and chloroform; and very slightly soluble in water. It has a melting point of about 130°C.
EXELDERM CREAM contains sulconazole nitrate, USP 10 mg/g in an emollient cream base consisting of propylene glycol, stearyl alcohol, isopropyl myristate, cetyl alcohol, polysorbate 60, sorbitan mono-stearate, glyceryl stearate (and) PEG-100 stearate, ascorbyl paImitate, and purified water, with sodium hydroxide and/or nitric acid added to adjust the pH.
-
CLINICAL PHARMACOLOGY
Sulconazole nitrate is an imidazole derivative with broad-spectrum antifungal activity that inhibits the growth in vitro of the common pathogenic dermatophytes including Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum and Microsporum canis. It also inhibits (in vitro) the organism responsible for tinea versicolor, Malassezia furfur. Sulconazole nitrate has been shown to be active in vitro against the following microorganisms, although clinical efficacy has not been established: Candida albicans and certain gram-positive bacteria.
A modified Draize test showed no allergic contact dermatitis and a phototoxicity study showed no phototoxic or photoallergic reaction to sulconazole nitrate cream. Maximization tests with sulconazole nitrate cream showed no evidence of contact sensitization or irritation.
-
INDICATIONS AND USAGE
EXELDERM (sulconazole nitrate, USP) CREAM, 1.0% is an antifungal agent indicated for the treatment of tinea pedis (athlete's foot), tinea cruris, and tinea corporis caused by Trichophyton rubrum, Trichophyton mentagro-phytes, Epidermophyton floccosum, and Microsporum canis,* and for the treatment of tinea versicolor.
*Efficacy for this organism in the organ system was studied in fewer than ten infections.
- CONTRAINDICATIONS
-
PRECAUTIONS
General
EXELDERM (sulconazole nitrate, USP) CREAM, 1.0% is for external use only. Avoid contact with the eyes. If irritation develops, the cream should be discontinued and appropriate therapy instituted.
Information for Patients
Patients should be told to use EXELDERM CREAM as directed by the physician, to use it externally only, and to avoid contact with the eyes.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to determine carcinogenic potential have not been performed. In vitro studies have shown no mutagenic activity.
Pregnancy
There are no adequate and well-controlled studies in pregnant women. Sulconazole nitrate should be used during pregnancy only if clearly needed. Sulconazole nitrate has been shown to be embryotoxic in rats when given in doses of 125 times the adult human dose (in mg/kg). The drug was not teratogenic in rats or rabbits at oral doses of 50 mg/kg/day.
Sulconazole nitrate given orally to rats at a dose 125 times the human dose resulted in prolonged gestation and dystocia. Several females died during the prenatal period, most likely due to labor complications.
Nursing Mothers
It is not known whether sulconazole nitrate is excreted in human milk. Caution should be exercised when sul- conazole nitrate is administered to a nursing woman.
Geriatric Use
Clinical studies of EXELDERM CREAM, 1.0%, did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients.
-
ADVERSE REACTIONS
There were no systemic effects and only infrequent cutaneous adverse reactions in 1185 patients treated with sulconazole nitrate cream in controlled clinical trials. Approximately 3% of these patients reported itching, 3% burning or stinging, and 1% redness. These complaints did not usually interfere with treatment.
-
CLINICAL STUDIES
In a vehicle-controlled study for the treatment of tinea pedis (moccasin type) due to T. rubrum, after 4 to 6 weeks of treatment 69% of patients on the active drug and 19% of patients on the drug vehicle had become KOH and culture negative. In addition, 68% of patients on the active drug and 20% of patients on the drug vehicle showed a good or excellent clinical response.
-
DOSAGE AND ADMINISTRATION
A small amount of cream should be gently massaged into the affected and surrounding skin areas once or twice daily, except in tinea pedis, where administration should be twice daily.
Early relief of symptoms is experienced by the majority of patients and clinical improvement may be seen fairly soon after treatment is begun; however, tinea corporis/cruris and tinea versicolor should be treated for 3 weeks and tinea pedis for 4 weeks to reduce the possibility of recurrence.
If significant clinical improvement is not seen after 4 to 6 weeks of treatment, an alternate diagnosis should be considered.
-
HOW SUPPLIED
EXELDERM (sulconazole nitrate, USP) CREAM, 1.0% is a smooth, glossy white to off-white cream having a slight characteristic odor. It is supplied as follows:
60 g tube – NDC 69489-711-60
Avoid excessive heat, above 40° C (104° F).
To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
JOURNEY®
MEDICAL CORPORATIONManufactured for:
Journey Medical Corp.
Scottsdale, AZ 85258
www.JMCderm.com141113
Revised March 2021 - Principal Display Panel – 60 g Carton Label
- Principal Display Panel – 60 gram Tube Label
-
INGREDIENTS AND APPEARANCE
EXELDERM
sulconazole nitrate creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69489-711 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sulconazole Nitrate (UNII: 1T89100D5U) (SULCONAZOLE - UNII:5D9HAA5Q5S) Sulconazole Nitrate 10 mg in 1 g Inactive Ingredients Ingredient Name Strength Propylene glycol (UNII: 6DC9Q167V3) Stearyl alcohol (UNII: 2KR89I4H1Y) Isopropyl myristate (UNII: 0RE8K4LNJS) Cetyl alcohol (UNII: 936JST6JCN) Polysorbate 60 (UNII: CAL22UVI4M) Sorbitan monostearate (UNII: NVZ4I0H58X) Glyceryl monostearate (UNII: 230OU9XXE4) PEG-100 stearate (UNII: YD01N1999R) Ascorbyl palmitate (UNII: QN83US2B0N) Water (UNII: 059QF0KO0R) Sodium hydroxide (UNII: 55X04QC32I) Nitric acid (UNII: 411VRN1TV4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69489-711-60 1 in 1 CARTON 06/07/2019 1 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018737 06/07/2019 Labeler - Journey Medical Corporation (079640860) Establishment Name Address ID/FEI Business Operations DPT Laboratories, Ltd. 832224526 ANALYSIS(69489-711) , MANUFACTURE(69489-711) , LABEL(69489-711) , PACK(69489-711)