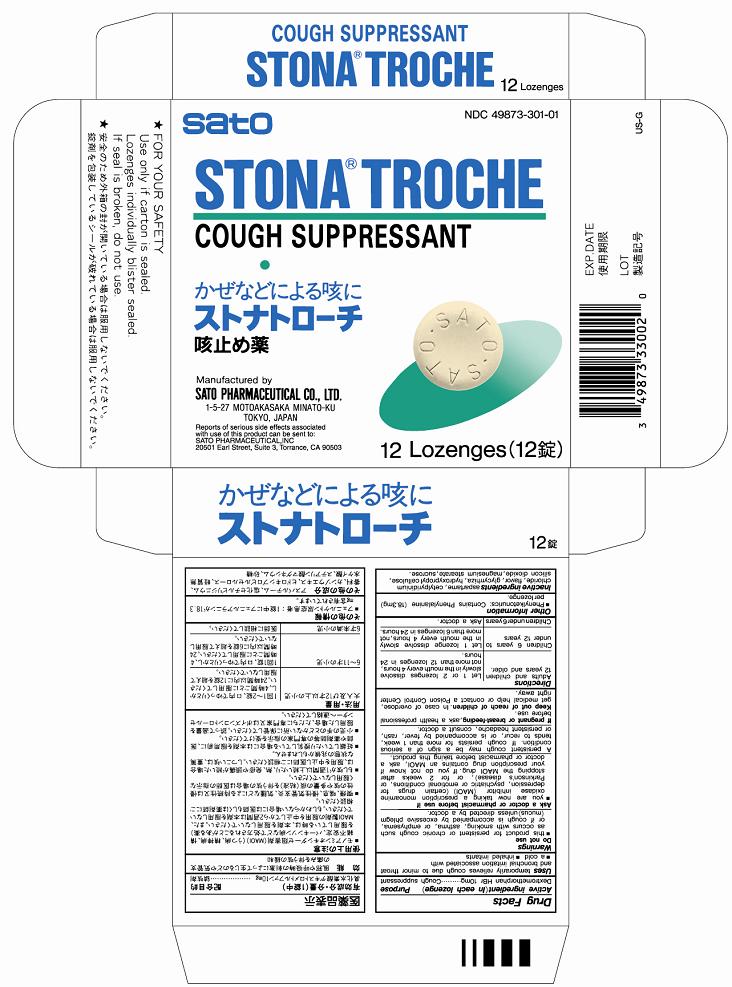
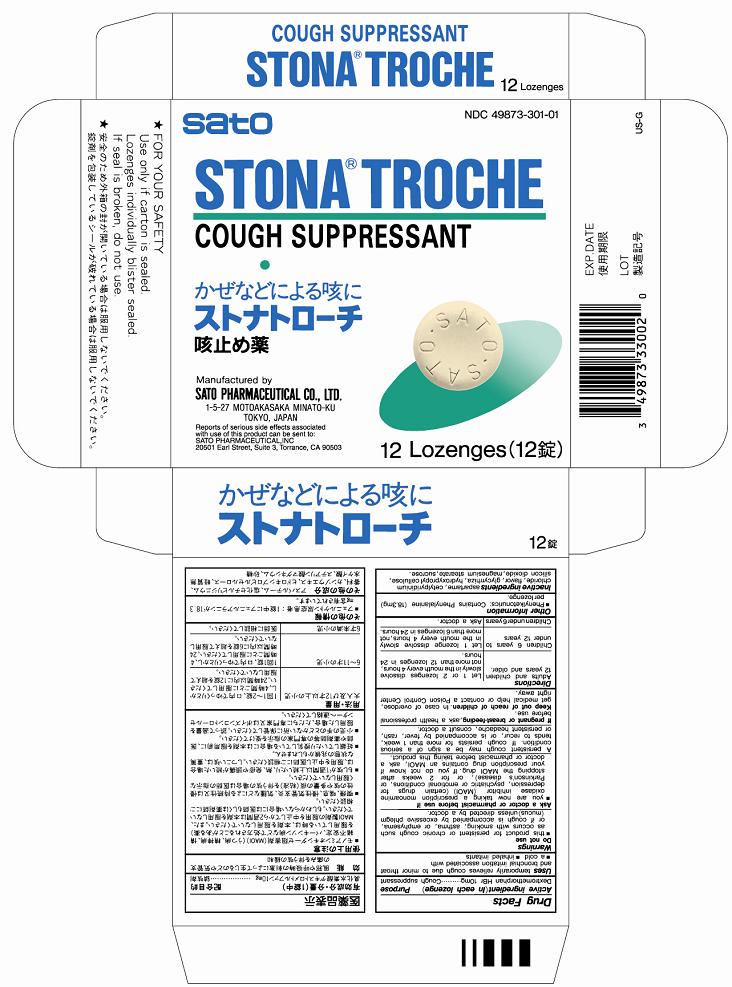
Label: STONA- dextromethorphan hydrobromide lozenge
- NDC Code(s): 49873-301-01
- Packager: Sato Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Enter section text here
Do not use
■this product for persistent or chronic cough such as occurs with smoking, asthema, or emphysem, or if cough is accompanied by excessive phlegm (mucus) unless directed by a doctor.Ask a doctor or pharmacist before use if
■you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product. A persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult a doctor. -
DOSAGE & ADMINISTRATION
Directions
Adults and children 12 years and older - Let 1 or 2 lozenges dissolve slowly in the mouth every 4 hours, not more than 12 lozenges in 24 hours.
Children 6 years to under 12 years - Let 1 lozenge dissolve slowly in the mouth every 4 hours, not more than 6 lozenges in 24 hours.
Children under 6 years - Ask a doctor
■once or twice daily, preferably morning and evening, when needed or as directed by a doctor
■start initial dosage with minimum dose, then adjust it to suit to bowel condition
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STONA
dextromethorphan hydrobromide lozengeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49873-301 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg Inactive Ingredients Ingredient Name Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) LICORICE (UNII: 61ZBX54883) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) SUCROSE (UNII: C151H8M554) ASPARTAME (UNII: Z0H242BBR1) Product Characteristics Color brown (pale brown) Score no score Shape ROUND Size 14mm Flavor BLUEBERRY, GRAPE Imprint Code SATO;SATO Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49873-301-01 1 in 1 CARTON 11/19/1995 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/19/1995 Labeler - Sato Pharmaceutical Co., Ltd. (690575642) Establishment Name Address ID/FEI Business Operations Sato Pharmaceutical Co., Ltd. 715699133 manufacture(49873-301) , label(49873-301) , pack(49873-301)