Label: BLEMISH CORRECTING SERUM SENSITIVE- salicylic acid gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 54272-103-11 - Packager: CEN BEAUTY LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 28, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
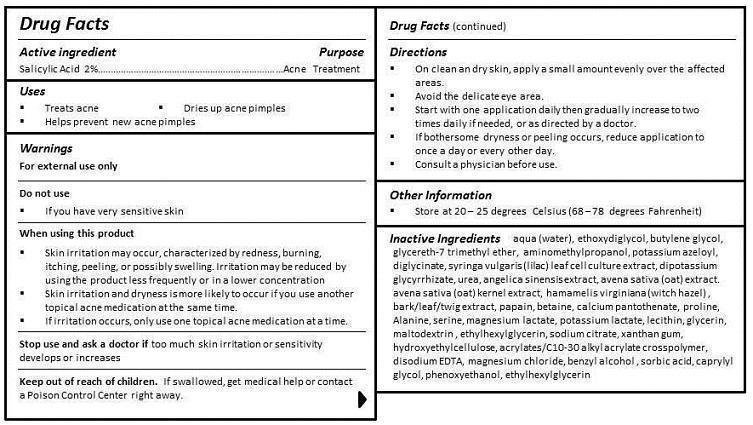
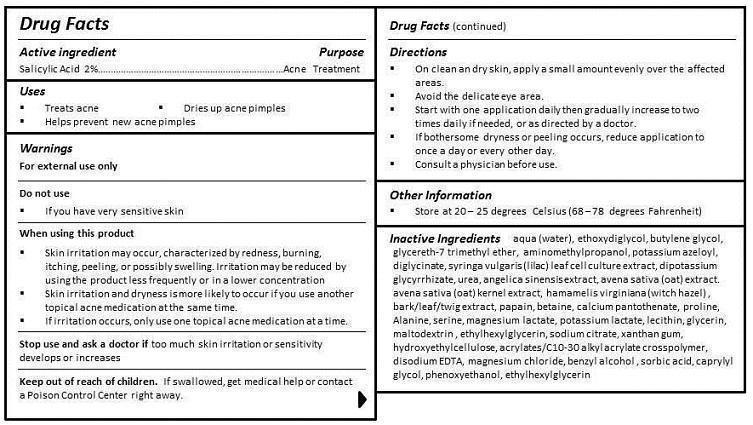
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
-
WHEN USING
WHEN USING THIS PRODUCT
- SKIN IRRITATION MAY OCCUR, CHARACTERIZED BY REDNESS, BURNING, ITCHING, PEELING, OR POSSIBLY SWELLING. IRRITATION MAY BE REDUCED BY USING THE PRODUCT LESS FREQUENTLY OR IN A LOWER CONCENTRATION.
- SKIN IRRITATION AND DRYNESS IS MORE LIKELY TO OCCUR IF YOU USE ANOTHER TOPICAL ACNE MEDICATION AT THE SAME TIME.
- IF IRRITATION OCCURS, ONLY USE ONE TOPICAL ACNE MEDICATION AT A TIME.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
DIRECTIONS
- ON CLEAN AND DRY SKIN, APPLY A SMALL AMOUNT EVENLY OVER THE AFFECTED AREAS.
- AVOID THE DELICATE EYE AREA.
- START WITH ONE APPLICATION DAILY THEN GRADUALLY INCREASE TO TWO TIMES DAILY IF NEEDED, OR AS DIRECTED BY A DOCTOR.
- IF BOTHERSOME DRYNESS OR PEELING OCCURS, REDUCE APPLICATION TO ONCE A DAY OR EVERY OTHER DAY.
- CONSULT A PHYSICIAN BEFORE USE.
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
INGREDIENTS
aqua (water), ethoxydiglycol, butylene glycol, glycereth-7 trimethyl ether, aminomethylpropanol, potassium azeloyl, diglycinate, syringa vulgaris (lilac) leaf cell culture extract, dipotassium glycyrrhizate, urea, angelica sinensis extract, avena sativa (oat) extract, avena sativa (oat) kernel extract, hamamelis virginiana (witch hazel) , bark/leaf/twig extract, papain, betaine, calcium pantothenate, proline, Alanine, serine, magnesium lactate, potassium lactate, lecithin, glycerin, maltodextrin , ethylhexylglycerin, sodium citrate, xanthan gum, hydroxyethylcellulose, acrylates/C10-30 alkyl acrylate crosspolymer, disodium EDTA, magnesium chloride, benzyl alcohol , sorbic acid, caprylyl glycol, phenoxyethanol, ethylhexylglycerin
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BLEMISH CORRECTING SERUM SENSITIVE
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54272-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERETH-7 TRIMETHYL ETHER (UNII: XMC7402M60) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) POTASSIUM AZELOYL DIGLYCINATE (UNII: N02RVN6NYP) SYRINGA VULGARIS WHOLE (UNII: U49SHU1VCI) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) UREA (UNII: 8W8T17847W) ANGELICA SINENSIS WHOLE (UNII: 697D19QDBN) AVENA SATIVA TOP (UNII: 1ZX4OXU3N6) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) PAPAIN (UNII: A236A06Y32) BETAINE (UNII: 3SCV180C9W) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) PROLINE (UNII: 9DLQ4CIU6V) ALANINE (UNII: OF5P57N2ZX) SERINE (UNII: 452VLY9402) MAGNESIUM LACTATE (UNII: MT6QI8324A) POTASSIUM LACTATE (UNII: 87V1KMK4QV) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) GLYCERIN (UNII: PDC6A3C0OX) MALTODEXTRIN (UNII: 7CVR7L4A2D) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM CITRATE (UNII: 1Q73Q2JULR) XANTHAN GUM (UNII: TTV12P4NEE) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) EDETATE DISODIUM (UNII: 7FLD91C86K) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) BENZYL ALCOHOL (UNII: LKG8494WBH) SORBIC ACID (UNII: X045WJ989B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54272-103-11 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 02/21/2013 Labeler - CEN BEAUTY LLC (078664118) Registrant - CEN BEAUTY LLC (078664118)