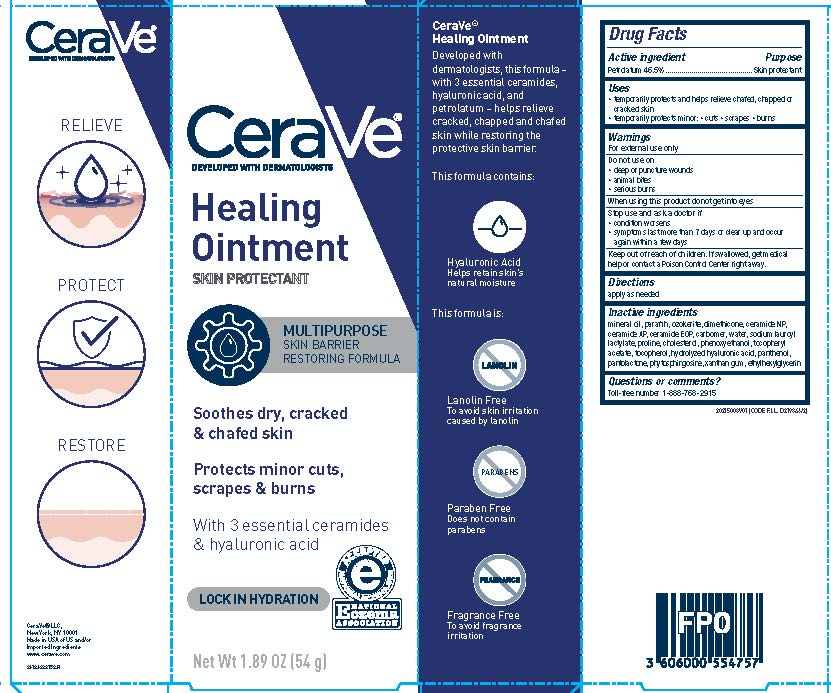
Label: CERAVE DEVELOPED WITH DERMATOLOGISTS HEALING- petrolatum ointment
-
NDC Code(s):
49967-500-01,
49967-500-02,
49967-500-03,
49967-500-04, view more49967-500-05, 49967-500-06, 49967-500-07, 49967-500-08
- Packager: L'Oreal USA Products Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use on
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
-
Inactive ingredients
mineral oil, paraffin, ozokerite, dimethicone, ceramide NP, ceramide AP, ceramide EOP, carbomer, water, sodium lauroyl lactylate, proline, cholesterol, phenoxyethanol, tocopheryl acetate, tocopherol, hydrolyzed hyaluronic acid, panthenol, pantolactone, phytosphingosine, xanthan gum, ethylhexylglycerin
- Questions?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CERAVE DEVELOPED WITH DERMATOLOGISTS HEALING
petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-500 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 46.5 g in 100 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PARAFFIN (UNII: I9O0E3H2ZE) CERESIN (UNII: Q1LS2UJO3A) DIMETHICONE (UNII: 92RU3N3Y1O) CERAMIDE 1 (UNII: 5THT33P7X7) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) WATER (UNII: 059QF0KO0R) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) LAUROYL PROLINE (UNII: HDI505B669) CHOLESTEROL (UNII: 97C5T2UQ7J) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) HYALURONIC ACID (UNII: S270N0TRQY) PANTHENOL (UNII: WV9CM0O67Z) PANTOLACTONE (UNII: J288D7O0JS) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-500-01 340 g in 1 JAR; Type 0: Not a Combination Product 09/20/2017 2 NDC:49967-500-02 85 g in 1 TUBE; Type 0: Not a Combination Product 09/20/2017 3 NDC:49967-500-03 144 g in 1 TUBE; Type 0: Not a Combination Product 09/20/2017 4 NDC:49967-500-04 15 in 1 TRAY 09/20/2017 4 2.5 g in 1 JAR; Type 0: Not a Combination Product 5 NDC:49967-500-05 10 g in 1 TUBE; Type 0: Not a Combination Product 09/20/2017 6 NDC:49967-500-06 2 in 1 CARTON 09/20/2017 6 10 g in 1 TUBE; Type 0: Not a Combination Product 7 NDC:49967-500-07 53.6 g in 1 TUBE; Type 0: Not a Combination Product 09/20/2017 8 NDC:49967-500-08 1 in 1 BLISTER PACK 09/20/2017 8 5 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 09/20/2017 Labeler - L'Oreal USA Products Inc. (002136794) Establishment Name Address ID/FEI Business Operations Accupac, Inc. 071609663 MANUFACTURE(49967-500)