Label: DOLUTEGRAVIR tablet, for suspension
- NDC Code(s): 65015-353-14, 65015-353-17, 65015-353-18
- Packager: Mylan Laboratories Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated March 5, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
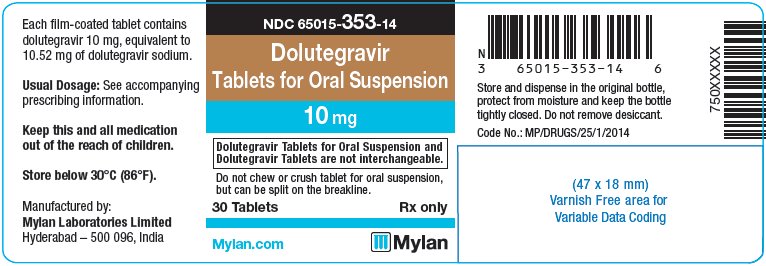
- PRINCIPAL DISPLAY PANEL – 10 mg
-
INGREDIENTS AND APPEARANCE
DOLUTEGRAVIR
dolutegravir tablet, for suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65015-353 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOLUTEGRAVIR SODIUM (UNII: 1Q1V9V5WYQ) (DOLUTEGRAVIR - UNII:DKO1W9H7M1) DOLUTEGRAVIR 10 mg Inactive Ingredients Ingredient Name Strength CALCIUM SULFATE DIHYDRATE (UNII: 4846Q921YM) CROSPOVIDONE (UNII: 2S7830E561) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE K30 (UNII: U725QWY32X) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) SUCRALOSE (UNII: 96K6UQ3ZD4) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color PINK Score 2 pieces Shape OVAL Size 12mm Flavor STRAWBERRY (strawberry cream) Imprint Code D;T;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65015-353-14 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/19/2020 2 NDC:65015-353-17 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/19/2020 3 NDC:65015-353-18 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/19/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 11/19/2020 Labeler - Mylan Laboratories Limited (650547156)