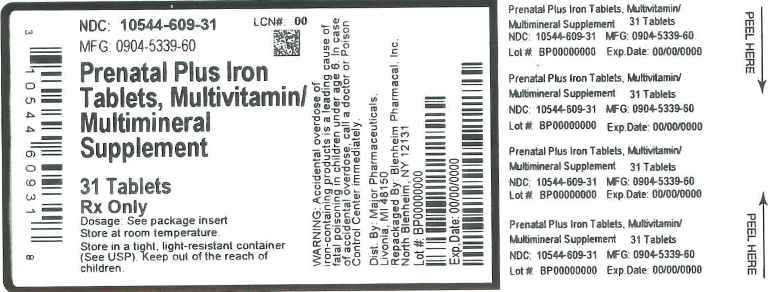
Label: PRENATAL PLUS IRON- multivitamin/multimineral supplement tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 10544-609-31 - Packager: Blenheim Pharmacal, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0904-5339
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 12, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Prenatal Plus Iron Tablet is caplet shaped, film coated tablet debossed CIS 28, and provides 10 vitamins and 4 minerals to supplement the diet before, during and after pregnancy.
Each Prenatal Plus tablet Contains:
Vitamin A (Acetate and Beta Carotene)............................................4000 I.U.
Vitamin C (Ascorbic acid)..................................................................120 mg
Vitamin D-3 (Cholecalciferol)...............................................................400 I.U.
Vitamin E (dl- Alpha Tocopheryl Acetate)...........................................22 I.U.
Thiamine (Vitamin B-1 from Thiamine Mononitrate...............................1.84 mg
Riboflavin (Vitamin B-2).......................................................................3 mg
Niacin(Niacinamide).............................................................................20 mg
Vitamin B-6 (Pyridoxine HCl)...............................................................10 mg
Folic Acid.............................................................................................1 mg
Vitamin B-12 (Cyanocobalamin)..........................................................12 mcg
Calcium (Calcium Carbonate)...............................................................200 mg
Iron (Carbonyl Iron)..............................................................................29 mg
Zinc (Zinc Oxide).................................................................................25 mg
Copper (Cupric Oxide)..........................................................................2 mg
Other ingredients: Ascorbyl Palmitate, Citric Acid anhydrous, DL-alpha Tocopherol, Ethylcellulose, FD&C Blue #2 lake, FD&C Red #40 lake, FD&C Yellow # 5 lake, FD&C Yello # 6 lake, Glucose, Gum Acacia, Hypromellose, Magnesium Stearate, Maize Starch, Maltodextrin, Methylcellulose, Microcrystalline Cellulose, Mineral Oil, Mono-and di-glycerides, Polyethyelene Glycol, Pregelatinized Corn Starch, Silicon Dioxide, Sorbic Acid, Soy Protein, Stearic Acid, Sucrose, Titanium Dioxide, Tricalcium Phosphate
- INDICATIONS
- DOSAGE
-
BOXED WARNING
(What is this?)
WARNING
Warning: Accidental overdose of iron containing products is a leading cause of fatal poisoning in children under age 6. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
Precautions: Folic acid may partially correct the hematological damage due to Vitamin B12 deficiency of pernicious anemia, while the associated neurological damage progesses.
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yello No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
- STORAGE
- HOW SUPPLIED
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PRENATAL PLUS IRON
multivitamin/multimineral supplement tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10544-609(NDC:0904-5339) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 3080 [iU] .BETA.-CAROTENE (UNII: 01YAE03M7J) (.BETA.-CAROTENE - UNII:01YAE03M7J) .BETA.-CAROTENE 920 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 22 [iU] THIAMINE MONONITRATE (UNII: 8K0I04919X) (Thiamine ION - UNII:4ABT0J945J) THIAMINE 1.84 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 10 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 200 mg IRON PENTACARBONYL (UNII: 6WQ62TAQ6Z) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 29 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 25 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) ALUMINUM OXIDE (UNII: LMI26O6933) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ASCORBYL PALMITATE (UNII: QN83US2B0N) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ETHYLCELLULOSE (100 MPA.S) (UNII: 47MLB0F1MV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) ACACIA (UNII: 5C5403N26O) MALTODEXTRIN (UNII: 7CVR7L4A2D) METHYLCELLULOSE (1500 MPA.S) (UNII: P0NTE48364) LIGHT MINERAL OIL (UNII: N6K5787QVP) CAPRYLIC/CAPRIC MONO/DIGLYCERIDES (UNII: U72Q2I8C85) TRICALCIUM PHOSPHATE (UNII: K4C08XP666) METHYL SORBATE (UNII: J3048615R1) SOY PROTEIN (UNII: R44IWB3RN5) Product Characteristics Color YELLOW (TAN) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code CIS28 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10544-609-31 31 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/17/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 05/17/2010 Labeler - Blenheim Pharmacal, Inc. (171434587) Registrant - Blenheim Pharmacal, Inc. (171434587) Establishment Name Address ID/FEI Business Operations Blenheim Pharmacal, Inc. 171434587 repack(10544-609)