Label: VALTRUM SOOTHING TOPICAL ANALGESIC- camphor, menthol ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 50786-003-03 - Packager: Drogefar C.A.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 12, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WHEN USING
- DO NOT USE
- SPL UNCLASSIFIED SECTION
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- INFORMATION FOR PATIENTS
-
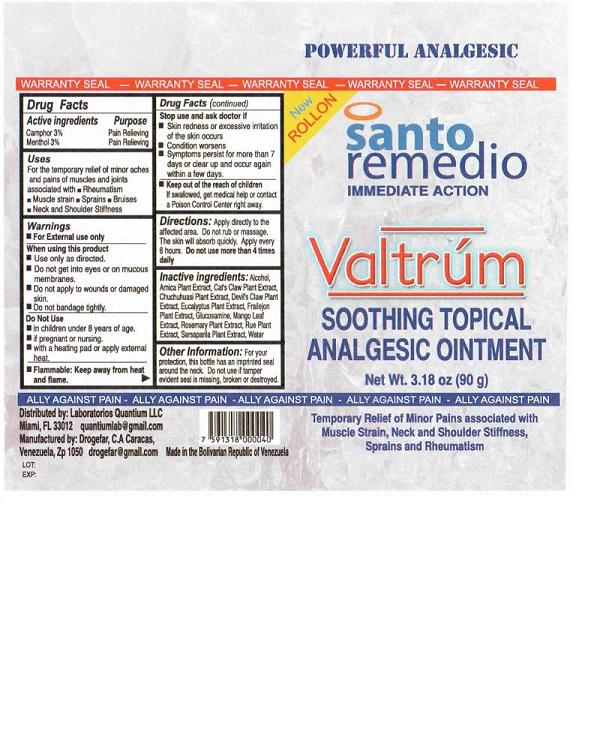
PRINCIPAL DISPLAY PANEL
POWERFUL ANALGESIC
WARRANTY SEAL - WARRANTY SEAL - WARRANTY SEAL - WARRANTY SEAL - WARRANTY SEAL
New ROLLON
santo remedio
IMMEDIATE ACTION
VALTRUM
Soothing Topical Analgesic Ointment
Net Wt. 3.18oz (90g)
ALLY AGAINST PAIN - ALLY AGAINST PAIN - ALLY AGAINST PAIN - ALLY AGAINST PAIN - ALLY AGAINST PAIN -
Distributed by Laboratorios Quantium LLC
Miami, FL 33012 quantiumlab@gmail.com
Manufactured by: Drogefar, C.A. Caracas,
Venezuela, Zp 1050
Lot:
Exp:
Made In the Bolivian Republic of Venezuela
Temporary relief of minor pains associated with Muscle Strain, Neck and Shoulder Stiffness, Sprains and Rheumatism
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VALTRUM SOOTHING TOPICAL ANALGESIC
camphor, menthol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50786-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 3 mg in 100 mg MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 3 mg in 100 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ARNICA MONTANA (UNII: O80TY208ZW) CAT'S CLAW (UNII: 9060PRM18Q) GLUCOSAMINE (UNII: N08U5BOQ1K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50786-003-03 90151.47 mg in 1 BOTTLE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 05/06/2010 Labeler - Drogefar C.A. (855055810) Registrant - Drogefar C.A. (855055810) Establishment Name Address ID/FEI Business Operations Drogefar C.A. 855055810 manufacture, label