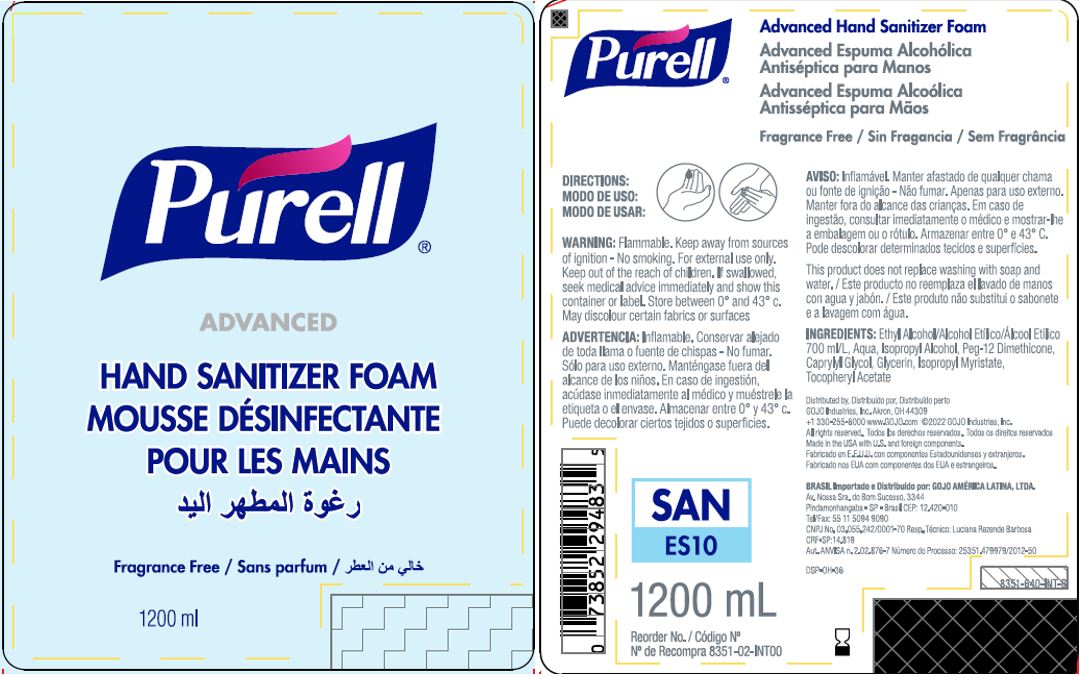
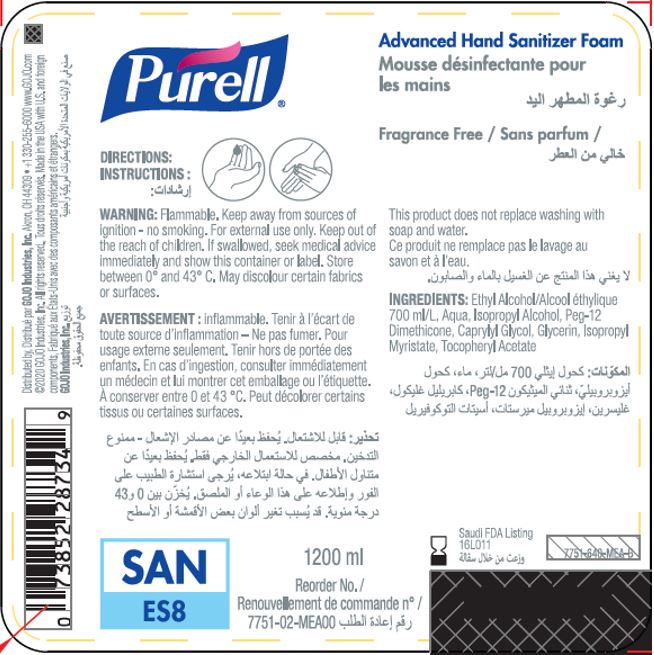
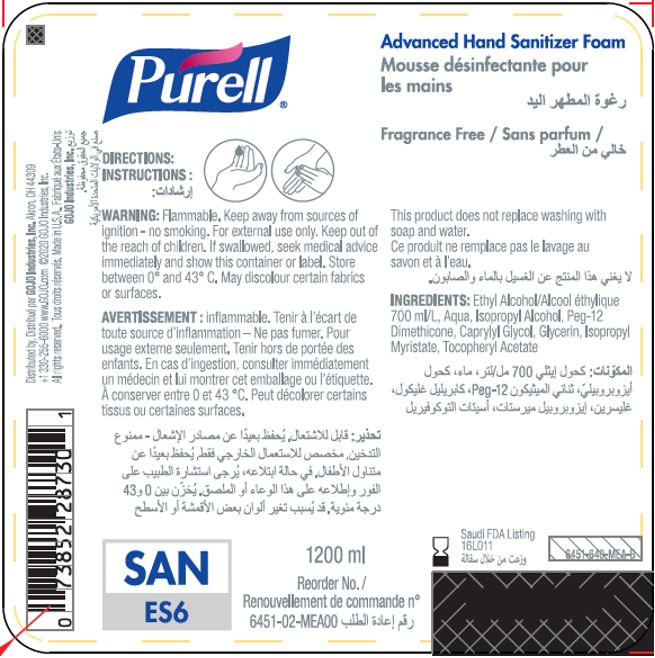
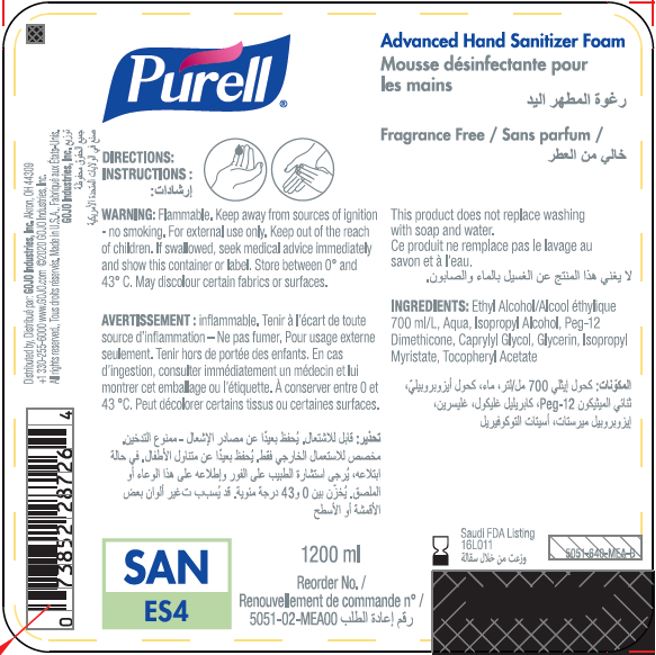
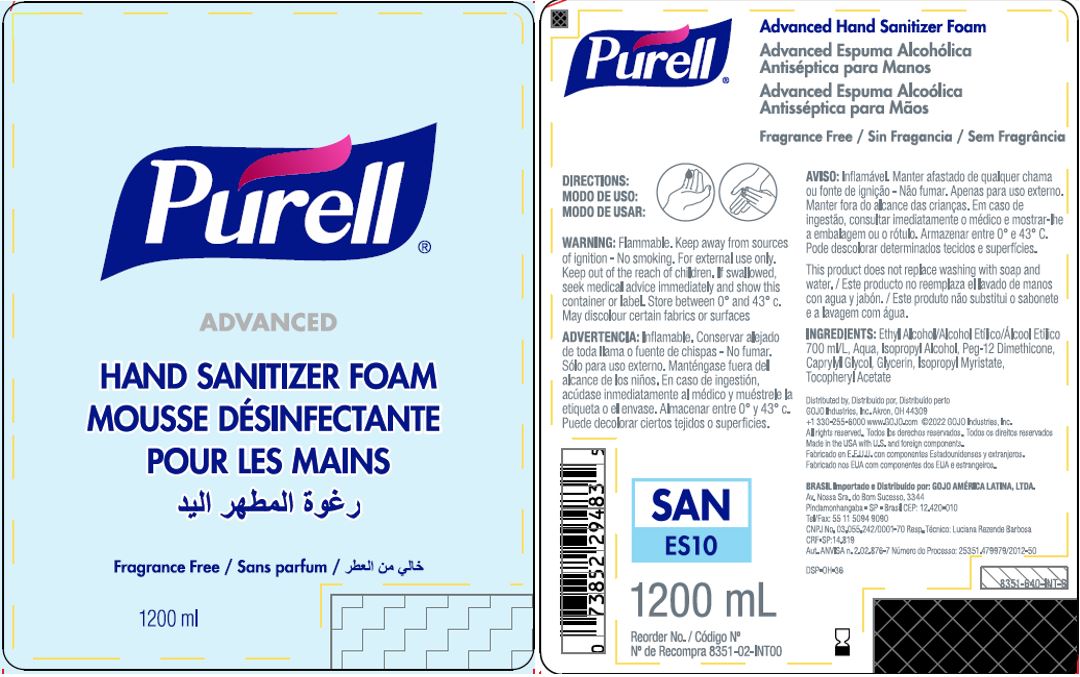
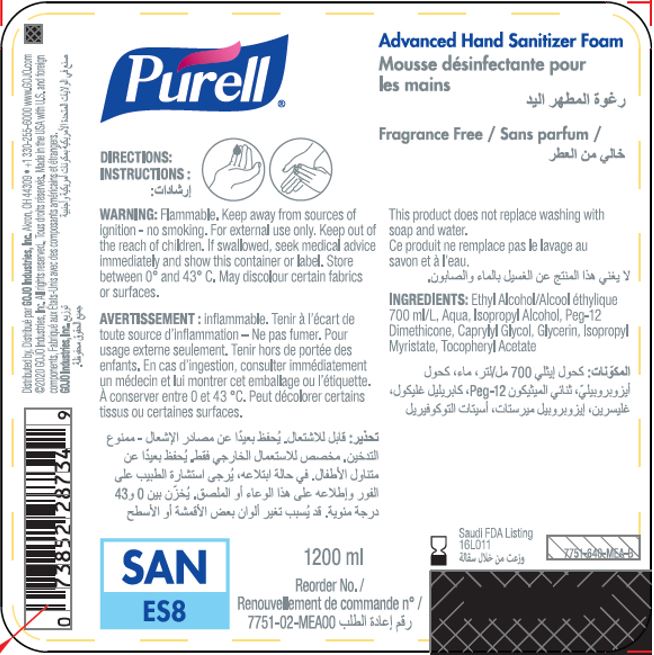
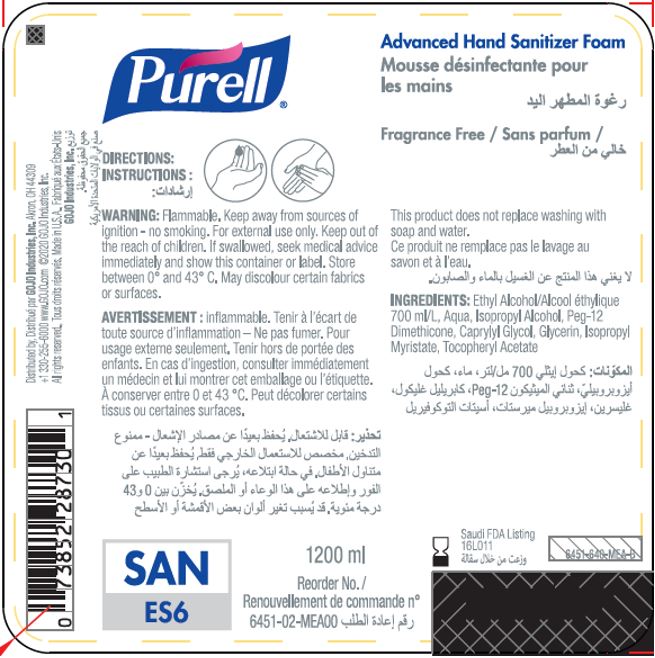
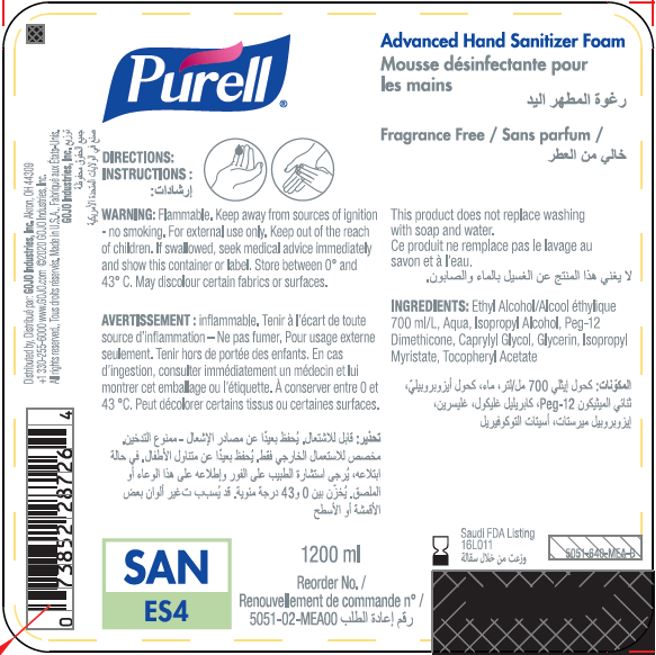
Label: PURELL ADVANCED HAND SANITIZER FOAM- alcohol liquid
- NDC Code(s): 21749-030-17, 21749-030-53, 21749-030-89, 21749-030-97
- Packager: GOJO Industries, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 24, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PURELL ADVANCED HAND SANITIZER FOAM
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21749-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Isopropyl Alcohol (UNII: ND2M416302) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21749-030-89 1200 mL in 1 PACKAGE; Type 0: Not a Combination Product 12/01/2014 2 NDC:21749-030-97 700 mL in 1 PACKAGE; Type 0: Not a Combination Product 12/01/2014 3 NDC:21749-030-53 535 mL in 1 PACKAGE; Type 0: Not a Combination Product 12/01/2014 4 NDC:21749-030-17 515 mL in 1 PACKAGE; Type 0: Not a Combination Product 01/10/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date export only 12/01/2014 Labeler - GOJO Industries, Inc. (004162038) Establishment Name Address ID/FEI Business Operations GOJO Industries, Inc. 036424534 manufacture(21749-030) , pack(21749-030) , label(21749-030)