Label: ISOSULFAN BLUE injection, solution
- NDC Code(s): 67457-220-00, 67457-220-05
- Packager: Mylan Institutional LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 16, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ISOSULFAN BLUE INJECTION 1% safely and effectively. See full prescribing information for ISOSULFAN BLUE INJECTION 1%.
ISOSULFAN BLUE injection 1% for subcutaneous use only
Initial U.S. Approval: 1981INDICATIONS AND USAGE
Isosulfan blue injection 1% upon subcutaneous administration, delineates the lymphatic vessels draining the region of injection. It is an adjunct to lymphography in: primary and secondary lymphedema of the extremities; chyluria, chylous ascites or chylothorax; lymph node involvement by primary or secondary neoplasm; lymph node response to therapeutic modalities (1.1).
DOSAGE AND ADMINISTRATION
Isosulfan blue injection 1% is to be administered subcutaneously, one-half (1/2) mL into three (3) interdigital spaces of each extremity per study. A maximum dose of 3 mL (30 mg) isosulfan blue is, therefore, injected (2.1).
DOSAGE FORMS AND STRENGTHS
1% aqueous solution (isosulfan blue)
CONTRAINDICATIONS
Hypersensitivity to triphenylmethane or related compounds (4).
WARNINGS AND PRECAUTIONS
- •
- Life threatening anaphylactic reactions have occurred after isosulfan blue injection 1% administration. Monitor patients closely for at least 60 minutes after administration of isosulfan blue injection 1% (5.1).
- •
- The admixture of isosulfan blue injection 1% with local anesthetics results in an immediate precipitation of 4% to 9% drug complex. Use a separate syringe for anesthetics (5.2).
- •
- Isosulfan blue injection 1% interferes with measurements in peripheral blood pulse oximetry. Arterial blood gas analysis may be needed (5.3).
ADVERSE REACTIONS
Hypersensitivity Reactions: Hypersensitivity reactions occur in approximately 2% of patients and include life threatening anaphylactic reactions with respiratory distress, shock, angioedema, urticaria, pruritus. A death has been reported following IV administration of a similar compound (6).
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
No drug interactions have been identified for isosulfan blue injection 1% (7).
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Lymphatic Vessel Delineation
2 DOSAGE AND ADMINISTRATION
2.1 Subcutaneous administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Precipitation of Isosulfan Blue Injection 1% by Lidocaine
5.3 Interference with Oxygen Saturation and Methemoglobin Measurements
6 ADVERSE REACTIONS
6.1 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.3 Nursing Mothers
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Teratogenic Effects
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Lymphatic Vessel Delineation
Isosulfan blue injection 1% upon subcutaneous administration, delineates lymphatic vessels draining the region of injection. It is an adjunct to lymphography in: primary and secondary lymphedema of the extremities; chyluria, chylous ascites or chylothorax; lymph node involvement by primary or secondary neoplasm; and lymph node response to therapeutic modalities.
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Life threatening anaphylactic reactions (respiratory distress, shock, angioedema) have occurred after isosulfan blue injection 1% administration. Reactions are more likely to occur in patients with a history of bronchial asthma, allergies, drug reactions or previous reactions to tri-phenylmethane dyes. Monitor patients closely for at least 60 minutes after administration of isosulfan blue injection 1%. Trained personnel should be available to administer emergency care including resuscitation.
5.2 Precipitation of Isosulfan Blue Injection 1% by Lidocaine
The admixture of isosulfan blue injection 1% (with local anesthetics (i.e. lidocaine)) in the same syringe results in an immediate precipitation of 4% to 9% drug complex. Use a separate syringe to administer a local anesthetic.
5.3 Interference with Oxygen Saturation and Methemoglobin Measurements
Isosulfan blue injection 1% interferes with measurements of oxygen saturation in peripheral blood by pulse oximetry and can cause falsely low readings. The interference effect is maximal at 30 minutes and minimal generally by 4 hours after administration. Arterial blood gas analysis may be needed to verify decreased arterial partial pressure of oxygen.
Isosulfan blue injection 1% may also cause falsely elevated readings of methemoglobin by arterial blood gas analyzer. Therefore, cooximetry may be needed to verify methemoglobin level.
-
6 ADVERSE REACTIONS
6.1 Postmarketing Experience
Hypersensitivity Reactions: Case series report an overall incidence of hypersensitivity reactions in approximately 2% of patients. Life threatening anaphylactic reactions have occurred. Manifestations include respiratory distress, shock, angioedema, urticaria, pruritus. A death has been reported following administration of a similar compound employed to estimate the depth of a severe burn. Reactions are more likely to occur in patients with a personal or family history of bronchial asthma, significant allergies, drug reactions or previous reactions to triphenylmethane dyes [see Warnings and Precautions (5)].
Laboratory Tests: Isosulfan blue injection 1% interferes with measurements of oxygen saturation by pulse oximetry and of methemoglobin by gas analyzer [see Warnings and Precautions (5)].
Skin: transient or long-term (tattooing) blue coloration.
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
-
11 DESCRIPTION
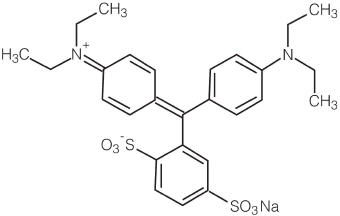
The chemical name of isosulfan blue injection 1% is N-[4- [[4-(diethylamino)phenyl] (2,5-disulfophenyl) methylene]-2,5-cyclohexadien-1-ylidene]-N-ethylethanaminium hydroxide, inner salt, sodium salt. Its structural formula is:
ISOSULFAN BLUE
Isosulfan blue injection 1% is a sterile aqueous solution for subcutaneous administration. Phosphate buffer in sterile, pyrogen free water is added in sufficient quantity to yield a final pH of 6.8 to 7.4. Each mL of solution contains 10 mg isosulfan blue, 6.6 mg sodium monohydrogen phosphate and 2.7 mg potassium dihydrogen phosphate. The solution contains no preservative. Isosulfan blue injection 1% is a contrast agent for the delineation of lymphatic vessels.
- 12 CLINICAL PHARMACOLOGY
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of isosulfan blue injection 1%. Reproduction studies in animals have not been conducted and, therefore, it is unknown if a problem concerning mutagenesis or impairment of fertility in either males or females exists.
13.2 Teratogenic Effects
Pregnancy Category C. Animal reproduction studies have not been conducted with isosulfan blue injection 1%. It is not known whether isosulfan blue injection 1% can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Isosulfan blue injection 1% should be given to a pregnant woman only if clearly needed.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Isosulfan Blue Injection 1% is supplied as a 5 mL single-dose vial, 1% aqueous solution in a phosphate buffer prepared by appropriate manufacturing to be sterile and pyrogen-free.
NDC 67457-220-05
carton containing 6 x 5 mL single-dose vialsStorage: Vials should be stored at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Avoid excessive heat.
Discard unused portion.
- 17 PATIENT COUNSELING INFORMATION
-
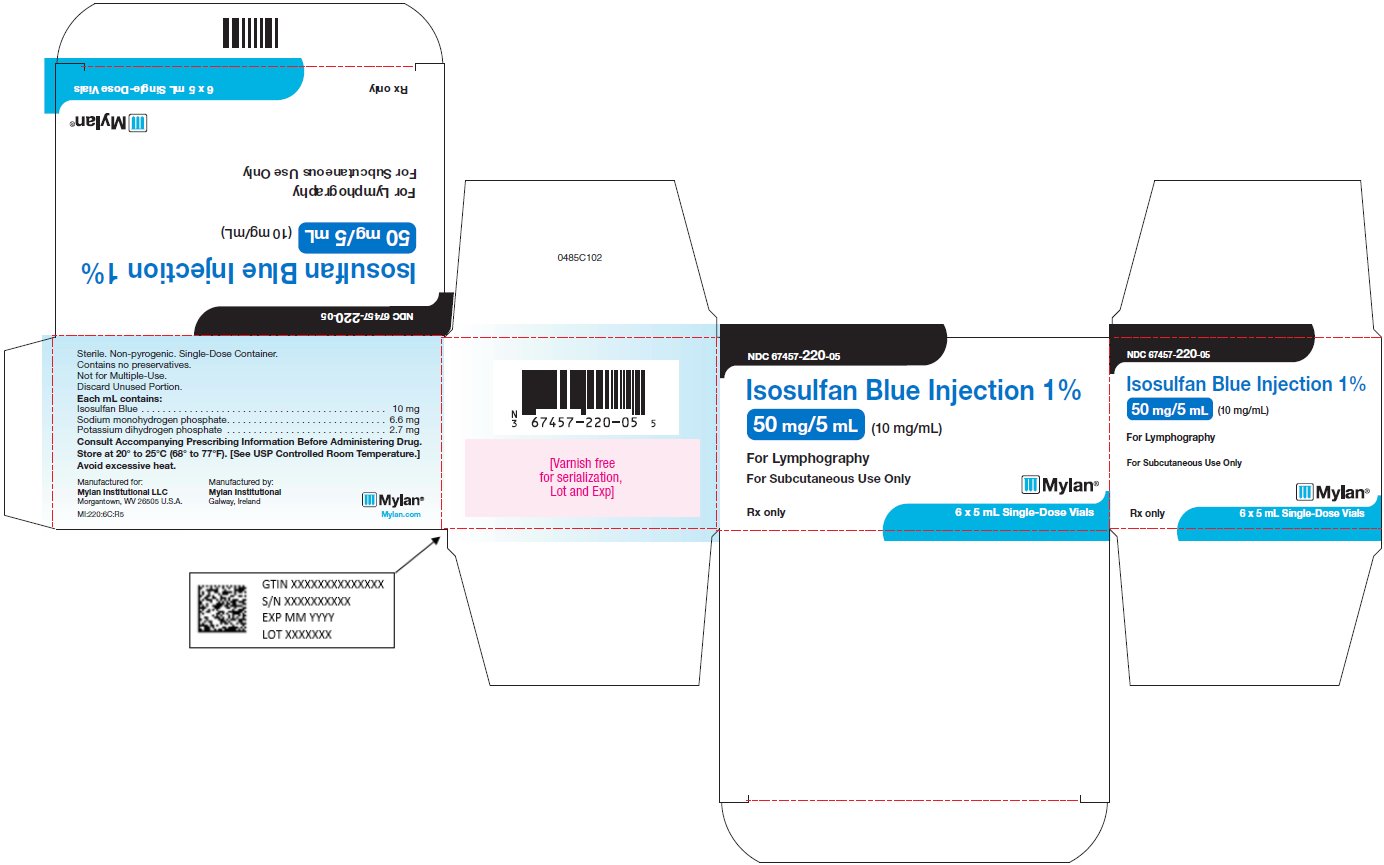
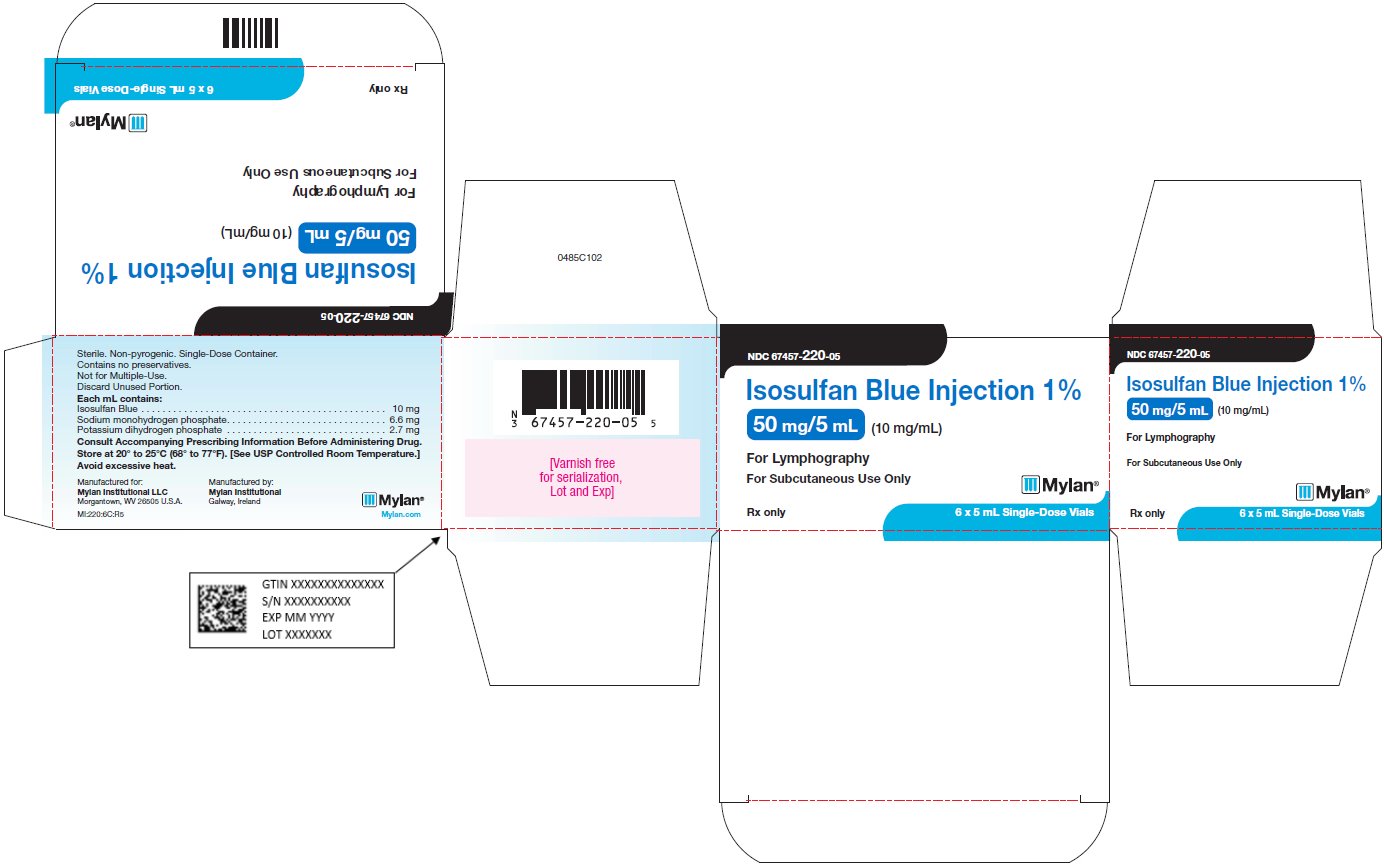
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 50 mg/5 mL
NDC 67457-220-05
Isosulfan Blue Injection 1%
50 mg/5 mL (10 mg/mL)For Lymphography
For Subcutaneous Use Only
Rx only 6 x 5 mL Single-Dose Vials
Sterile. Non-Pyrogenic. Single-Dose Container.
Contains no preservatives.
Not for Multiple-Use.
Discard Unused Portion.Each mL contains:
Isosulfan Blue . . . . . . . . . . . . . . . . . . . . . . . . . 10 mg
Sodium monohydrogen phosphate. . . . . . . . 6.6 mg
Potassium dihydrogen phosphate . . . . . . . . 2.7 mgConsult Accompanying Prescribing Information Before Administering Drug.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Avoid excessive heat.
Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.Manufactured by:
Mylan Institutional
Galway, IrelandMI:220:6C:R5
Mylan.com
-
INGREDIENTS AND APPEARANCE
ISOSULFAN BLUE
isosulfan blue injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67457-220 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOSULFAN BLUE (UNII: 39N9K8S2A4) (ISOSULFAN BLUE INNER SALT - UNII:NS6Q291771) ISOSULFAN BLUE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 6.6 mg in 1 mL POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) 2.7 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67457-220-05 6 in 1 CARTON 03/14/2013 1 NDC:67457-220-00 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090874 03/14/2013 Labeler - Mylan Institutional LLC (790384502)