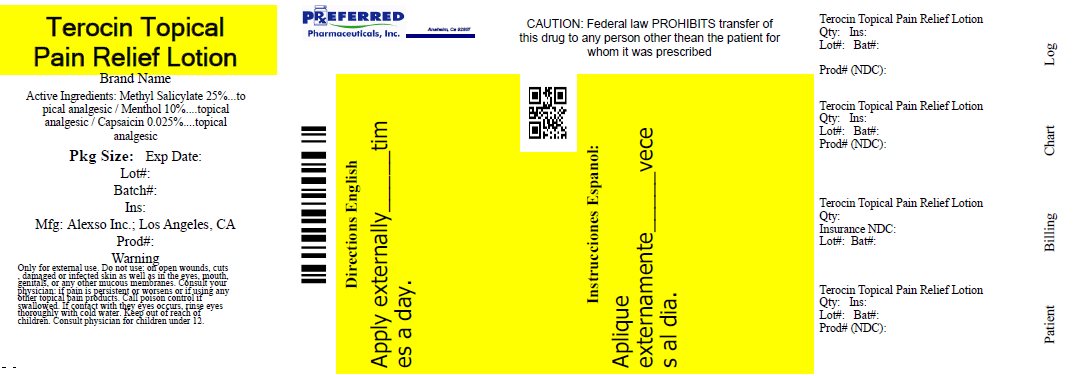
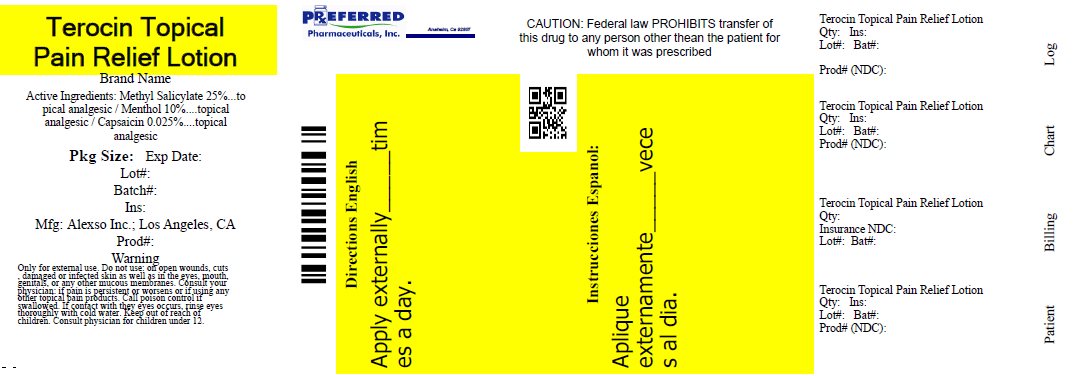
Label: NEW TEROCIN- methyl salicylate, capsaicin, and menthol lotion
- NDC Code(s): 68788-7821-1
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 50488-1129
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients:
- Purpose
- Uses:
- Warnings
- Directions:
-
Inactive Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Borago Officinalis (Borage) Seed Oil, Boswellia Serrata Extract, C12-15 Alkyl Benzoate, Cetearyl Alcohol, Cetyl Alcohol, Diazolidinyl Urea, Dimethyl Sulfone (DMSO), DMDM Hydantoin, Glyceryl Stearate, Lavandula Angustifolia (Lavender) Oil, Methyl Paraben, PEG-100 Stearate, Polysorbate-20, Polysorbate-60, Propyl Paraben, Propylene Glycol, Stearic Acid, Stearyl Alcohol, Triethanolamine, Xanthan Gum.
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NEW TEROCIN
methyl salicylate, capsaicin, and menthol lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-7821(NDC:50488-1129) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 25 g in 100 mL CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.025 g in 100 mL MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 10 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CETYL ALCOHOL (UNII: 936JST6JCN) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) LIDOCAINE (UNII: 98PI200987) LAVENDER OIL (UNII: ZBP1YXW0H8) ALOE VERA LEAF (UNII: ZY81Z83H0X) BORAGE SEED OIL (UNII: F8XAG1755S) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) XANTHAN GUM (UNII: TTV12P4NEE) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) DMDM HYDANTOIN (UNII: BYR0546TOW) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-7821-1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/16/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 11/16/2020 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 RELABEL(68788-7821)