Label: DIPHENHYDRAMINE HYDROCHLORIDE liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 62320-010-05, 62320-010-10 - Packager: Plastikon Healthcare, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 7, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
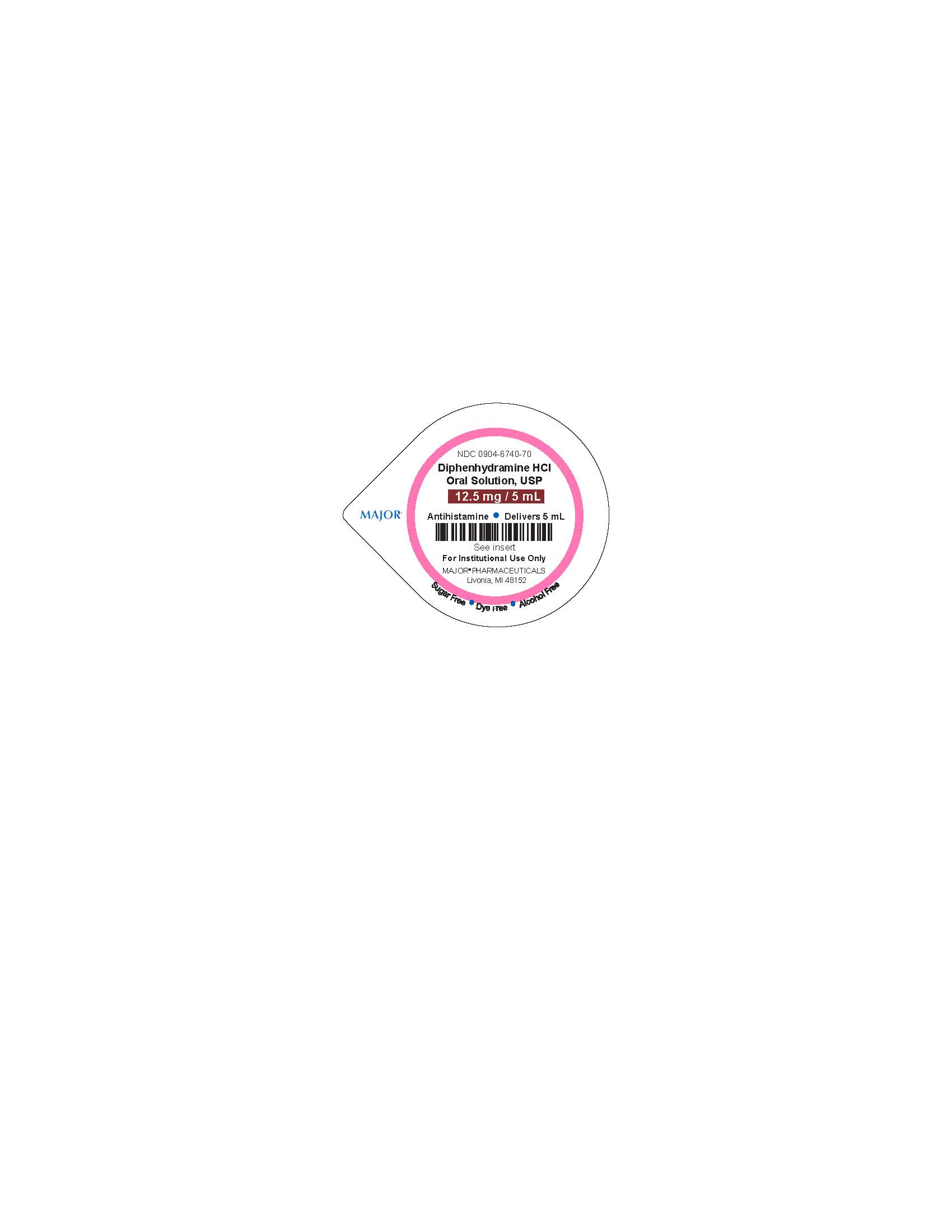
Diphenhydramine HCl 12.5 mg/ 5 mL Cups

 NDC 0904-6740-70
NDC 0904-6740-70
Diphenhydramine HCl
Oral Solution, USP
12.5 mg/5 mL
Antihistamine - Delivers 5 mL
See Insert
For Instituional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 64152
Sugar Free - Dye Free - Alcohol Free
Diphenhydramine HCl 12.5 mg/5 mL
Major Pharmaceuticals - IFU - Institutional Use OnlyDirections
• Use the following dosage guidelines when using this product
Age (yr)
Dose (mL)
adults and children 12 years and over
take 10 mL every 4 to 6 hours; not more than 60 mL in 24 hours
children 6 years to under 12 years
take 5 mL every 4 to 6 hours; not more than 30 mL in 24 hours
children under 6 years
ask a doctor

Diphenhydramine HCl 12.5 mg/5 mL
Major Pharmaceuticals - IFU - For Institutional Use OnlyWarnings
Do not use
in neonates or premature infants
if pregnant or breast-feeding
if hypersensitive to diphenhydramine HCl and other similar antihistamines
with any other product containing diphenhydramine, even one used on skin
to make a child sleepy
___________________________________________________________________
Ask a doctor before use if you have
glaucoma a breathing problem such as emphysema or chronic bronchitis
a sodium restricted diet trouble urinating due to an enlarged prostate gland
___________________________________________________________________
Ask a doctor or pharmacist before use if
taking tranquilizers or sedatives
___________________________________________________________________
When using this product
marked drowsiness may occur avoid alcoholic drinks
alcohol, sedatives, and tranquilizers may increase drowsiness
be careful when driving a motor vehicle or operating machinery
excitability may occur, especially in children
___________________________________________________________________
Diphenhydramine HCl 12.5 mg/5 mL
Major Pharmaceuticals - IFU - for Institutional Use OnlyInactive ingredients cherry flavor, citric acid, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucralose
Diphenhydramine HCl 12.5 mg/5 mL
Major Pharmaceuticals - IFU - For Institutional Use OnlyUses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: • runny nose • sneezing • itchy, watery eyes • itchy throat
Diphenydramine HCl 12.5 mg/ 5 mL
Major Pharmaceutical - IFU - For Institutional Use OnlyKeep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Diphenhydramine HCl 12.5 mg/ 5 mL
Major Pharmaceuticals - IFU - For Intitutional Use OnlyAntihistamine
Diphenhydramine HCl 12.5 mg/ 5 mL
Major Pharmaceuticals - IFU - For Institutional Use OnlyActive ingredient (in each 5 mL cup) Purpose Diphenhydramine HCl USP 12.5 mg..………………………………………Antihistamine
Diphenhydramine HCl 12.5 mg/5 mL
Major Pharmaceuticals - IFU - For Institutional Use OnlyOther information
- each 5 mL contains: sodium 15 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
Diphenhydramine HCl 12.5 mg/ 5 mL
Major Pharmaceuticals - IFU - For Institutional use Only
Product Insert
Diphenhydramine HCl Oral Solution, USP
NDC 0904-6740-70
10 x 5 mL Unit Dose Cups
Active ingredient (in each 5 mL cup) Purpose Diphenhydramine HCl USP 12.5 mg..………………………………………Antihistamine
Uses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itchy throat
Warnings
Do not use
- in neonates or premature infants
- if pregnant or breast-feeding
- if hypersensitive to diphenhydramine HCl and other similar antihistamines
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- a sodium restricted diet
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if
- taking tranquilizers or sedatives
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Other information
- each 5 mL contains: sodium 15 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
Inactive ingredients cherry flavor, citric acid, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucralose
Directions
Use the following dosage guidelines when using this product
Age (yr) Dose (mL)
adults and children 12 years and over take 10 mL every 4 to 6 hours; not more than 60 mL in 24 hours
children 6 years to under 12 years take 5 mL every 4 to 6 hours; not more than 30 mL in 24 hours
children under 6 years ask a doctor
Questions or comments?
Call 1-800-616-2471
Re-order No. 700900
MAJOR® PHARMACEUTICALS
17177 N Laurel Park Dr., Suite 233
Livonia, MI 48152
-
Diphenhydramine HCl 25 mg / 10 mL Cups

 NDC 0904-6741-72
NDC 0904-6741-72
Diphenhydramine HCl
Oral Solution, USP
25 mg/10 mL
Antihistamine - Delivers 10 mL
See Insert
For Instituional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 64152
Sugar Free - Dye Free - Alcohol Free
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - for Instutional use OnlyDirections
Use the following dosage guidelines when using this product
Age (yr) Dose (mL)
adults and children 12 years and over take 10 mL every 4 to 6 hours; not more than 60 mL in 24 hours
children 6 years to under 12 years ask a doctor
Diphenhydramine HCl 10 mg/ 10 mL
Major Pharmaceuticals - For Institutional Use OnlyWarnings
Do not use
• in neonates or premature infants
• if pregnant or breast-feeding
• if hypersensitive to diphenhydramine HCl and other similar antihistamines
• with any other product containing diphenhydramine, even one used on skin
• to make a child sleepy
___________________________________________________________________
Ask a doctor before use if you have
• glaucoma • a breathing problem such as emphysema or chronic bronchitis
• a sodium restricted diet • trouble urinating due to an enlarged prostate gland
___________________________________________________________________
Ask a doctor or pharmacist before use if
• taking tranquilizers or sedatives
___________________________________________________________________
When using this product
• marked drowsiness may occur • avoid alcoholic drinks
• alcohol, sedatives, and tranquilizers may increase drowsiness
• be careful when driving a motor vehicle or operating machinery
• excitability may occur, especially in children
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - For Institutional Use OnlyInactive ingredients cherry flavor, citric acid, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucralose
Diphenhydramine HCl 10 mg / 10 mL
Major Pharmaceuticals - For Institutional Use OnlyUses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: • runny nose • sneezing • itchy, watery eyes • itchy throat
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - For Institutional use OnlyKeep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - For Institutional Use OnlyActive ingredient (in each 10 mL cup) Purpose Diphenhydramine HCl USP 25 mg..………………………………………Antihistamine
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - IFU - For Institutional Use Only• each 10 mL contains: sodium 30 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
Diphenhydramine HCl 25 mg/ 10 mL
Major Pharmaceuticals - IFU - For Institutional Use Only roduct Insert
roduct Insert
Diphenhydramine HCl Oral Solution, USP
NDC 0904-6741-72
10 x 10 mL Unit Dose Cups
Active ingredient (in each 10 mL cup) Purpose Diphenhydramine HCl USP 25 mg..………………………………………Antihistamine
Uses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itchy throat
Warnings
Do not use
- in neonates or premature infants
- if pregnant or breast-feeding
- if hypersensitive to diphenhydramine HCl and other similar antihistamines
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- a sodium restricted diet
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if
- taking tranquilizers or sedatives
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
Use the following dosage guidelines when using this product
Age (yr) Dose (mL)
adults and children 12 years and over take 10 mL every 4 to 6 hours; not more than 60 mL in 24 hours
children 6 years to under 12 years ask a doctor
Other information
- each 10 mL contains: sodium 30 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
Inactive ingredients cherry flavor, citric acid, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucralose
Questions or comments?
Call 1-800-616-2471
Re-order
No. 700901
MAJOR® PHARMACEUTICALS
17177 N Laurel Park Dr., Suite 233
Livonia, MI 48152
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62320-010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) SUCRALOSE (UNII: 96K6UQ3ZD4) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) POLOXAMER 407 (UNII: TUF2IVW3M2) GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62320-010-05 10 in 1 CASE 12/04/2018 1 10 in 1 TRAY 1 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 2 NDC:62320-010-10 10 in 1 CASE 12/04/2018 2 10 in 1 TRAY 2 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 12/04/2018 Labeler - Plastikon Healthcare, LLC (041717941) Registrant - Plastikon Healthcare, LLC (041717941) Establishment Name Address ID/FEI Business Operations Plastikon Healthcare, LLC 041717941 manufacture(62320-010)