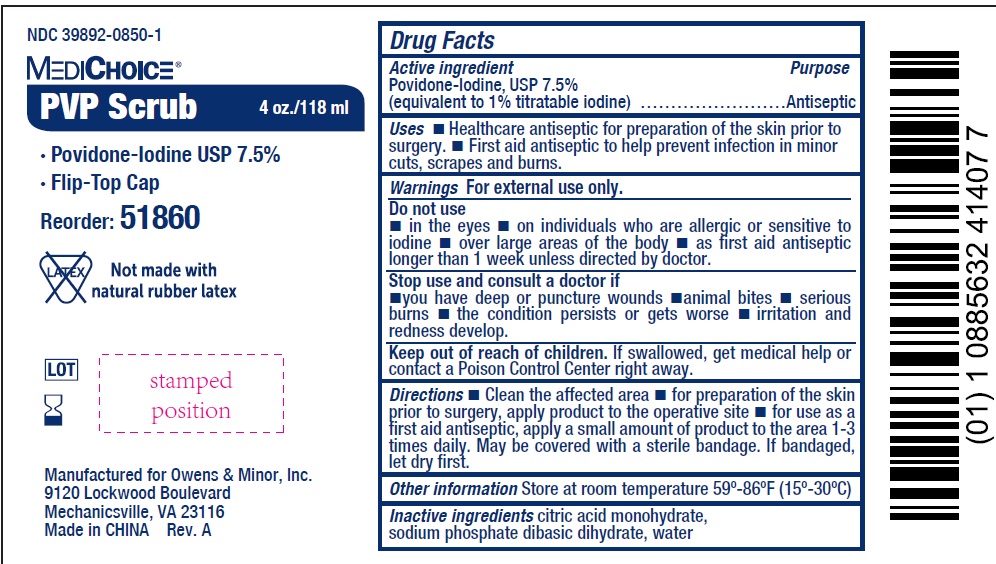
Label: MEDICHOICE PVP SCRUB- povidone-iodine solution
- NDC Code(s): 39892-0850-1, 39892-0850-2
- Packager: Owens & Minor Distribution, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
-
Warnings
For external use only.
Do not use
- in the eyes
- on individuals who are allergic or sensitive to iodine
- over large areas of the body
- as first aid antiseptic longer than 1 week unless directed by doctor.
- Directions
- Other information
- Inactive ingredients
- Pacakge Labeling:
- Pacakge Labeling:
-
INGREDIENTS AND APPEARANCE
MEDICHOICE PVP SCRUB
povidone-iodine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:39892-0850 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 75 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:39892-0850-1 118 mL in 1 PACKAGE; Type 0: Not a Combination Product 01/25/2024 2 NDC:39892-0850-2 48 in 1 CASE 01/25/2024 2 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/25/2024 Labeler - Owens & Minor Distribution, Inc. (847412269)