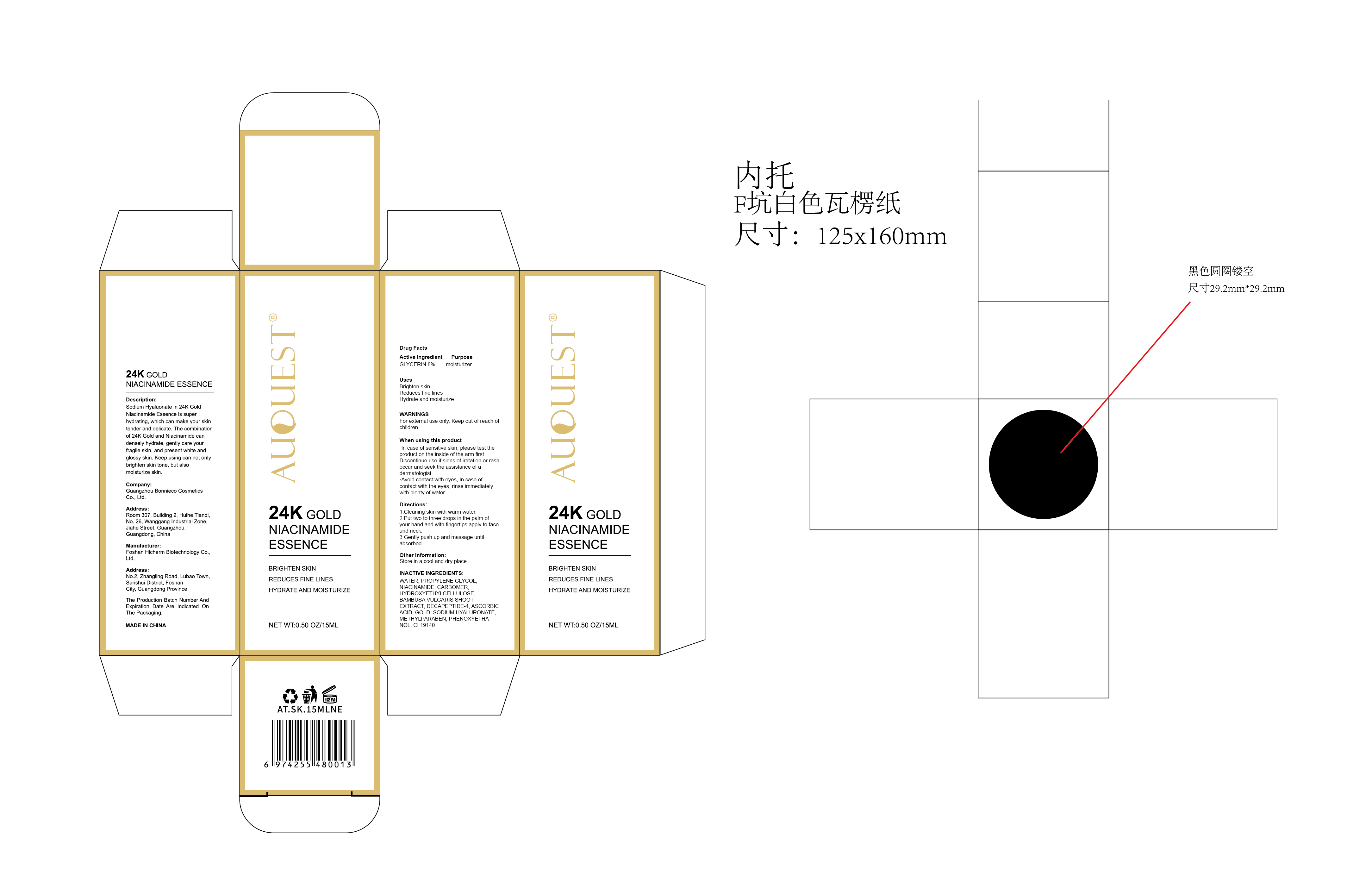
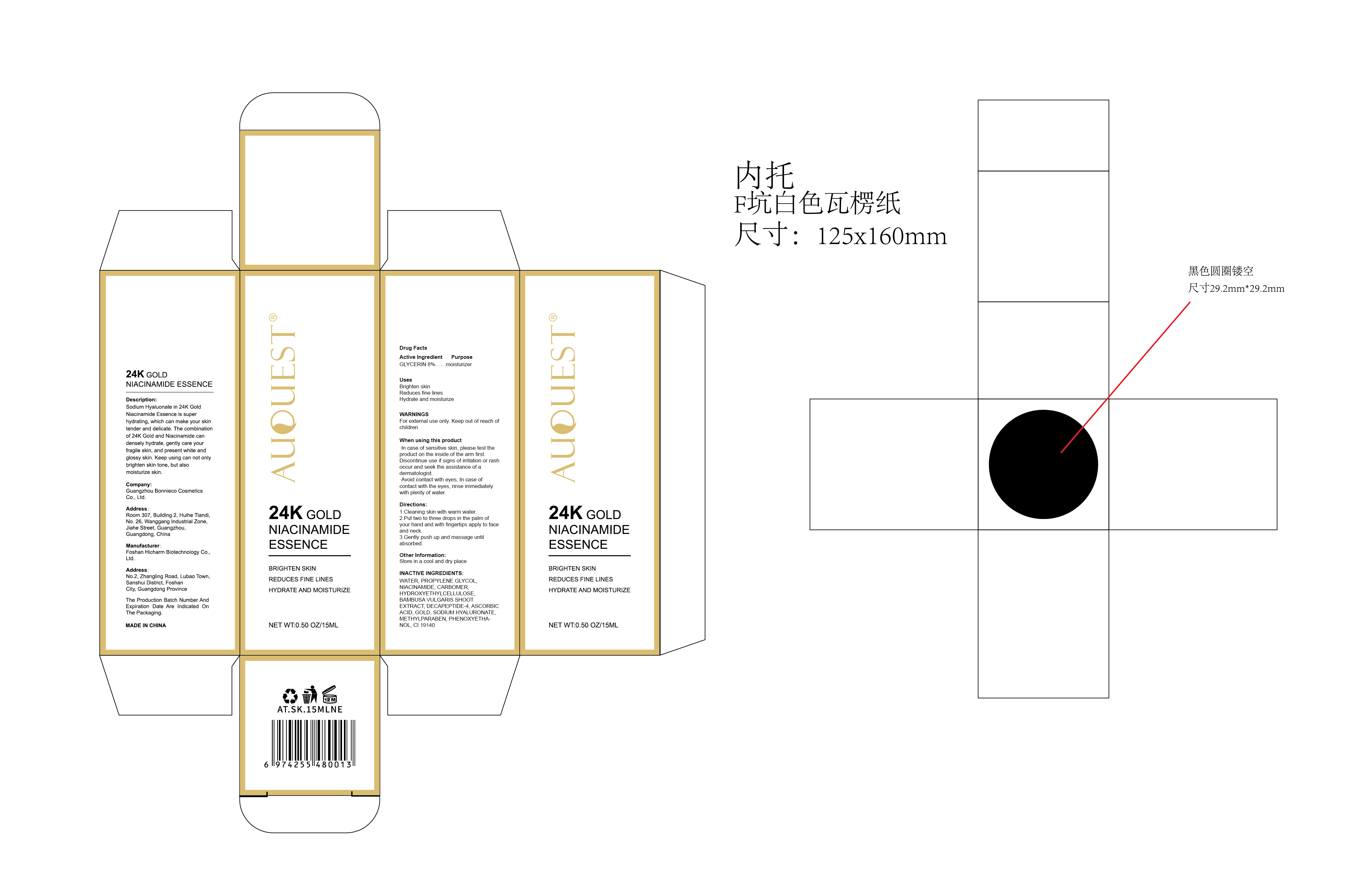
Label: AUQUEST 24K GOLD NIACINAMIDE ESSENCE- 24k gold niacinamide essence liquid
- NDC Code(s): 84186-001-01
- Packager: Guangzhou Bonnieco Cosmetics Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- When Using
- Keep Oot Of Reach Of Children
- Directions
-
Other information
Description:
Sodium Hyaluonate in 24K GoldNiacinamide Essence is super hydrating, which can make your skin tender and delicate. The combination of 24K Gold and Niacinamide can densely hydrate, gently care your fragile skin, and present white and glossy skin. Keep using can not only brighten skin tone, but also moisturize skin. - Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AUQUEST 24K GOLD NIACINAMIDE ESSENCE
24k gold niacinamide essence liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84186-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 8 g in 100 mL Inactive Ingredients Ingredient Name Strength GOLD (UNII: 79Y1949PYO) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) ASCORBIC ACID (UNII: PQ6CK8PD0R) HYALURONATE SODIUM (UNII: YSE9PPT4TH) WATER (UNII: 059QF0KO0R) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) BAMBUSA VULGARIS STEM (UNII: SMR633LHTC) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) ACETYL DECAPEPTIDE-3 (UNII: 30510A08G7) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) NIACINAMIDE (UNII: 25X51I8RD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84186-001-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 03/19/2024 Labeler - Guangzhou Bonnieco Cosmetics Co., Ltd (412244189) Establishment Name Address ID/FEI Business Operations Guangzhou Bonnieco Cosmetics Co., Ltd 412244189 label(84186-001) , manufacture(84186-001)