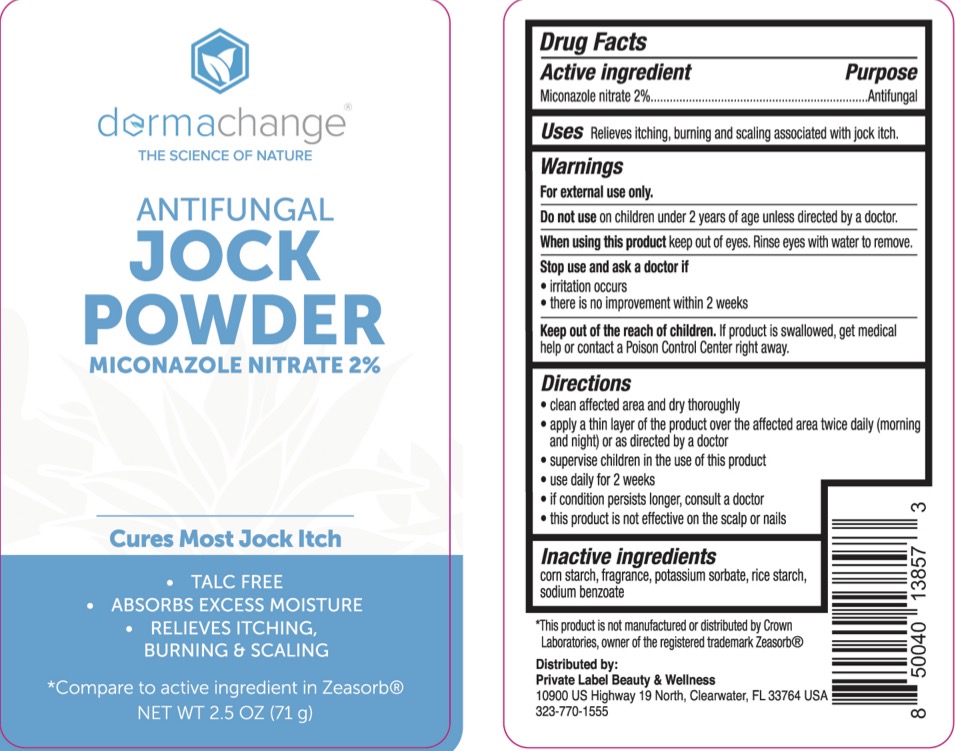
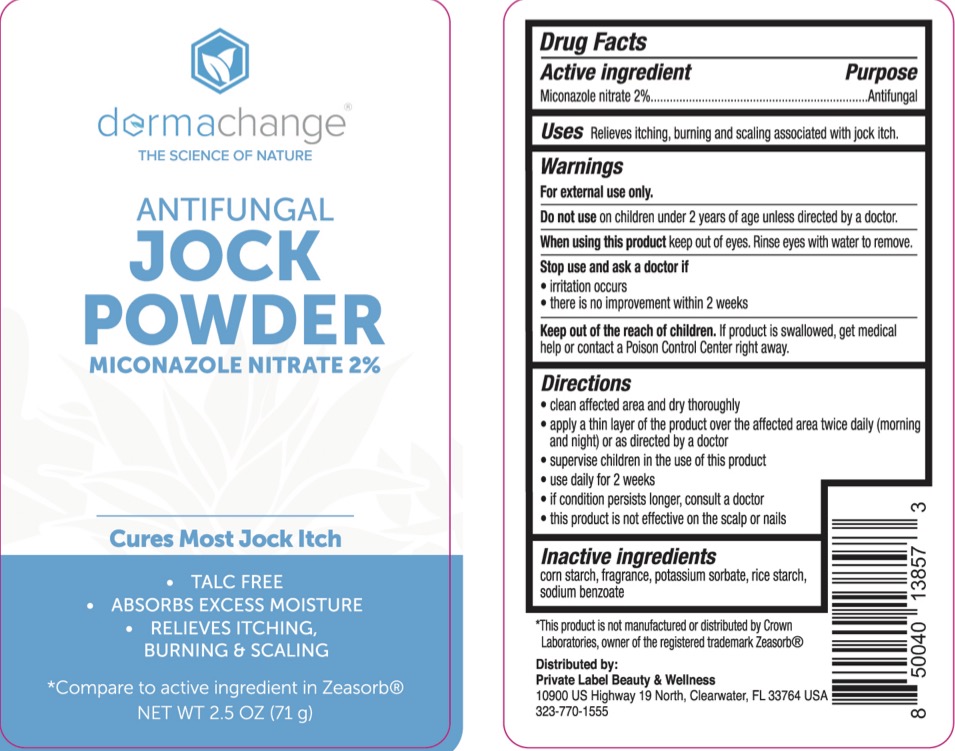
Label: DERMACHANGE JOCK- miconazole nitrate powder
- NDC Code(s): 83520-961-02, 83520-961-21
- Packager: Private label Beauty and Wellness
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Jock Powder
Cleanse the affected area and dry thoroughly. Apply a thin layer of the product over the affected area twice daily or as directed by a doctor.
Supervise children in the use of this product.
Use daily for 2 weeks.
If the condition persists, consult a doctor.
This product is not effective on the scalp or nails.
Foot Powder
Cleanse the affected area and dry thoroughly. Apply a thin layer of the product over the affected area twice daily or as directed by a doctor.
For athlete's foot: pay special attention to space between toes; wear well-fitting ventilated shoes and change socks at least once daily.
Supervise children in the use of this product.
Use daily for 4 weeks.
If the condition persists, consult a doctor.
This product is not effective on the scalp or nails.
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DERMACHANGE JOCK
miconazole nitrate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83520-961 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 2 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) STARCH, CORN (UNII: O8232NY3SJ) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) STARCH, RICE (UNII: 4DGK8B7I3S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83520-961-21 71 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/25/2023 2 NDC:83520-961-02 71 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/25/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 10/25/2023 Labeler - Private label Beauty and Wellness (035014854) Registrant - Derma Care Research Labs, LLC (116817470) Establishment Name Address ID/FEI Business Operations Derma Care Research Labs, LLC 116817470 manufacture(83520-961)