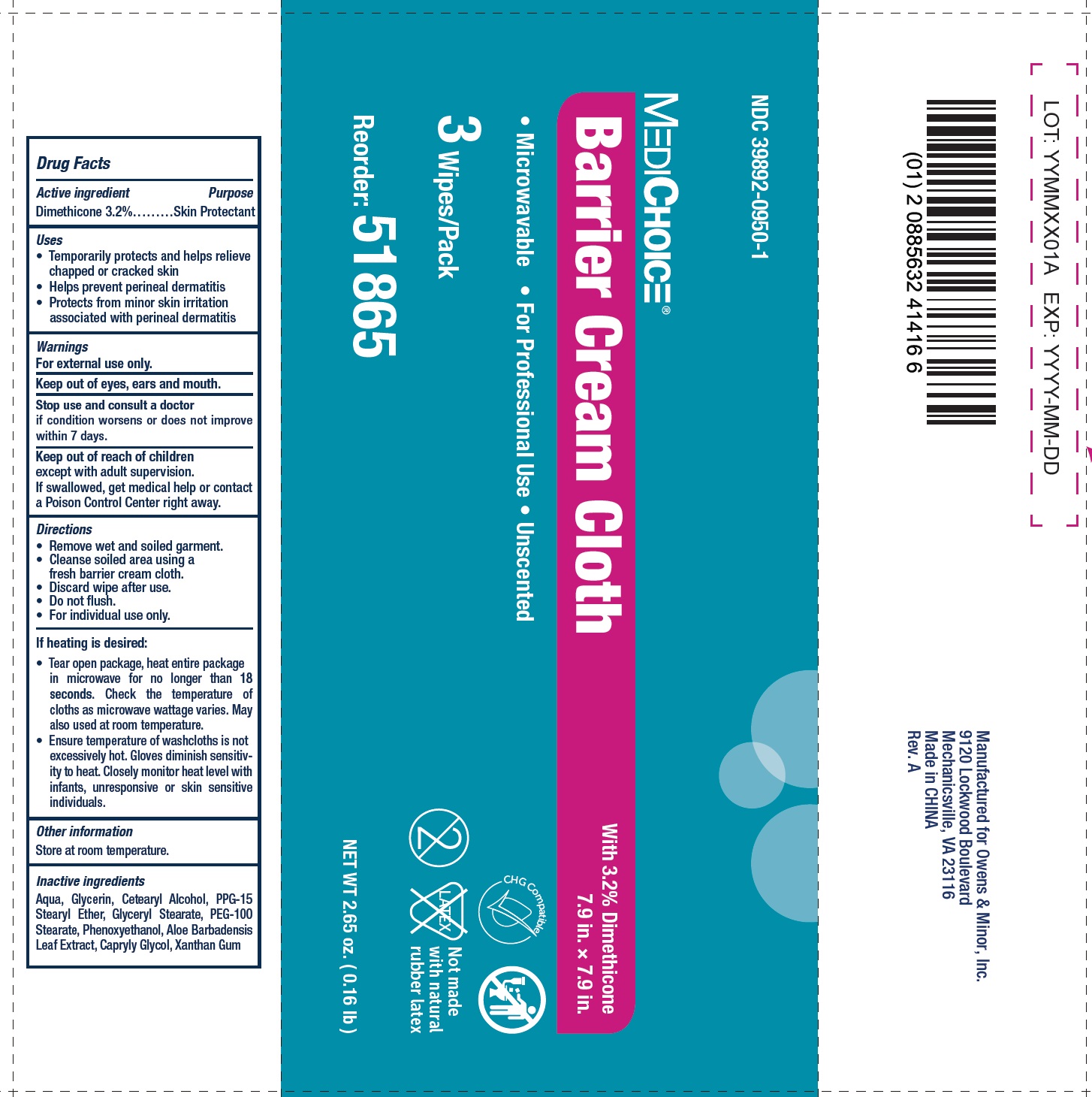
Label: MEDICHOICE BARRIER- dimethicone cloth
- NDC Code(s): 39892-0950-1, 39892-0950-2
- Packager: Owens & Minor Distribution, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
-
If heating is desired:
• Tear open package, heat entire package in microwave for no longer than 18 seconds. Check the temperature of cloths as microwave wattage varies. May also used at room temperature. • Ensure temperature of washcloths is not excessively hot. Gloves diminish sensitivity to heat. Closely monitor heat level with infants, unresponsive or skin sensitive individuals.
- Inactive ingredients
- Package Labeling:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
MEDICHOICE BARRIER
dimethicone clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:39892-0950 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 32 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PPG-15 STEARYL ETHER (UNII: 1II18XLS1L) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:39892-0950-1 3 in 1 PACKAGE 01/25/2024 1 20 g in 1 PATCH; Type 0: Not a Combination Product 2 NDC:39892-0950-2 72 in 1 CASE 01/25/2024 2 216 in 1 PACKAGE 2 20 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 01/25/2024 Labeler - Owens & Minor Distribution, Inc. (847412269)