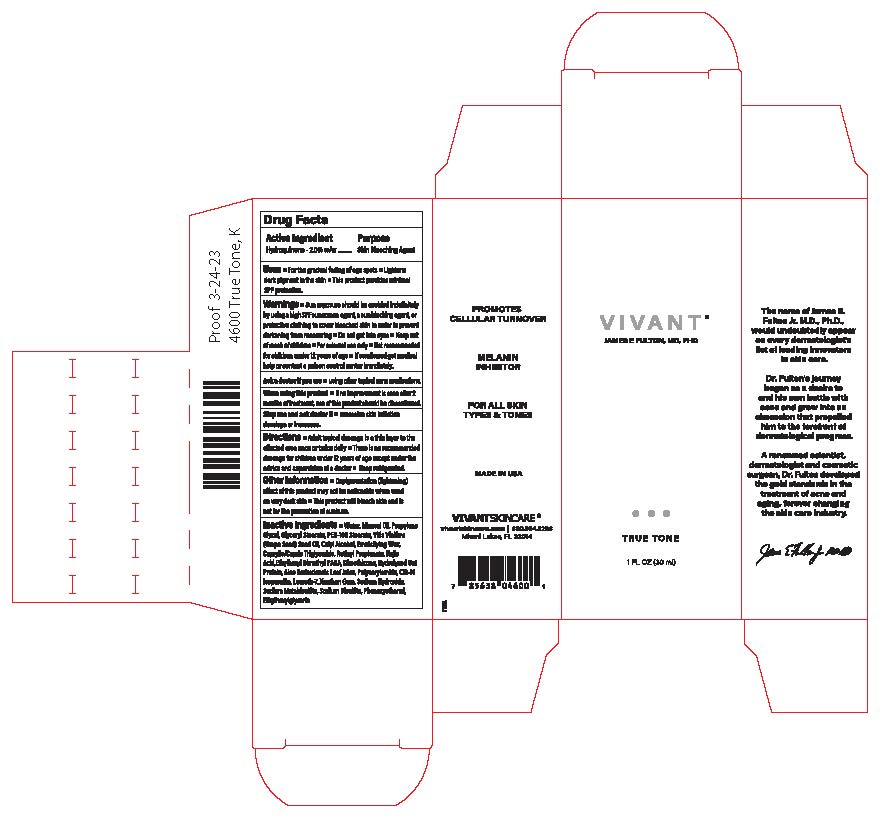
Label: VIVANT TRUE TONE- hydroquinone cream
- NDC Code(s): 63750-002-01
- Packager: Vivant Pharmaceuticals, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Indication
- Uses
-
Warnings
Warnings ■Sun exposure should be avoided indefinitely by using a high SPF sunscreen agent, a sunblocking agent, or protective clothing to cover bleached skin in order to prevent darkening from reoccurring ■ Do not get into eyes ■Keep out of reach of
children ■For external use only ■Not recommended for children under 12 years of age ■ If swallowed get medical help or contact a poison control center immediately.
- Ask a doctor if you are:
- Directions
-
Inactive Ingredients
Inactive Ingredients• Water, Mineral Oil, Propylene Glycol, Glyceryl Stearate, PEG-10O Stearate, Vitis Vinifera (Grape Seed) Seed Oil, Cetyl Alcohol, Emulsifying Wax, Caprylic/Capric Triglyceride, Retinyl Propionate, Kojic Acid, EthylhexylDimethyl PABA, Dimethicone, HydrolyzedOat Protein, Aloe Barbadensis Leaf Juice, Polyacrylamide, C13-14 isoparaffin, Laureth-7, Xanthan Gum, Sodium Hydroxide, Sodium Metabisulfite, Sodium Bisullte, Phenoxyethanol, Ethylhexylglycerin
- When using this product
- Stop use and ask the doctor if
- Warnings
- Other Information
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
VIVANT TRUE TONE
hydroquinone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63750-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM METABISULFITE (UNII: 4VON5FNS3C) PADIMATE O (UNII: Z11006CMUZ) VITIS VINIFERA LEAF OIL (UNII: 76K69S9H5Z) CETYL ALCOHOL (UNII: 936JST6JCN) WATER (UNII: 059QF0KO0R) KOJIC ACID (UNII: 6K23F1TT52) ALOE VERA LEAF (UNII: ZY81Z83H0X) RETINYL PROPIONATE (UNII: 32JK994WMC) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) AVENA SATIVA LEAF (UNII: 206PI19V7R) SODIUM HYDROXIDE (UNII: 55X04QC32I) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SODIUM BISULFITE (UNII: TZX5469Z6I) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL 1-STEARATE (UNII: 258491E1RZ) PEG-60 STEARATE (UNII: 7TB32G765E) POLYSORBATE 60 (UNII: CAL22UVI4M) MINERAL OIL (UNII: T5L8T28FGP) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63750-002-01 1 in 1 BOX 02/01/2024 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2024 Labeler - Vivant Pharmaceuticals, LLC (782696814) Establishment Name Address ID/FEI Business Operations Vivant Pharmaceuticals, LLC 782696814 manufacture(63750-002)