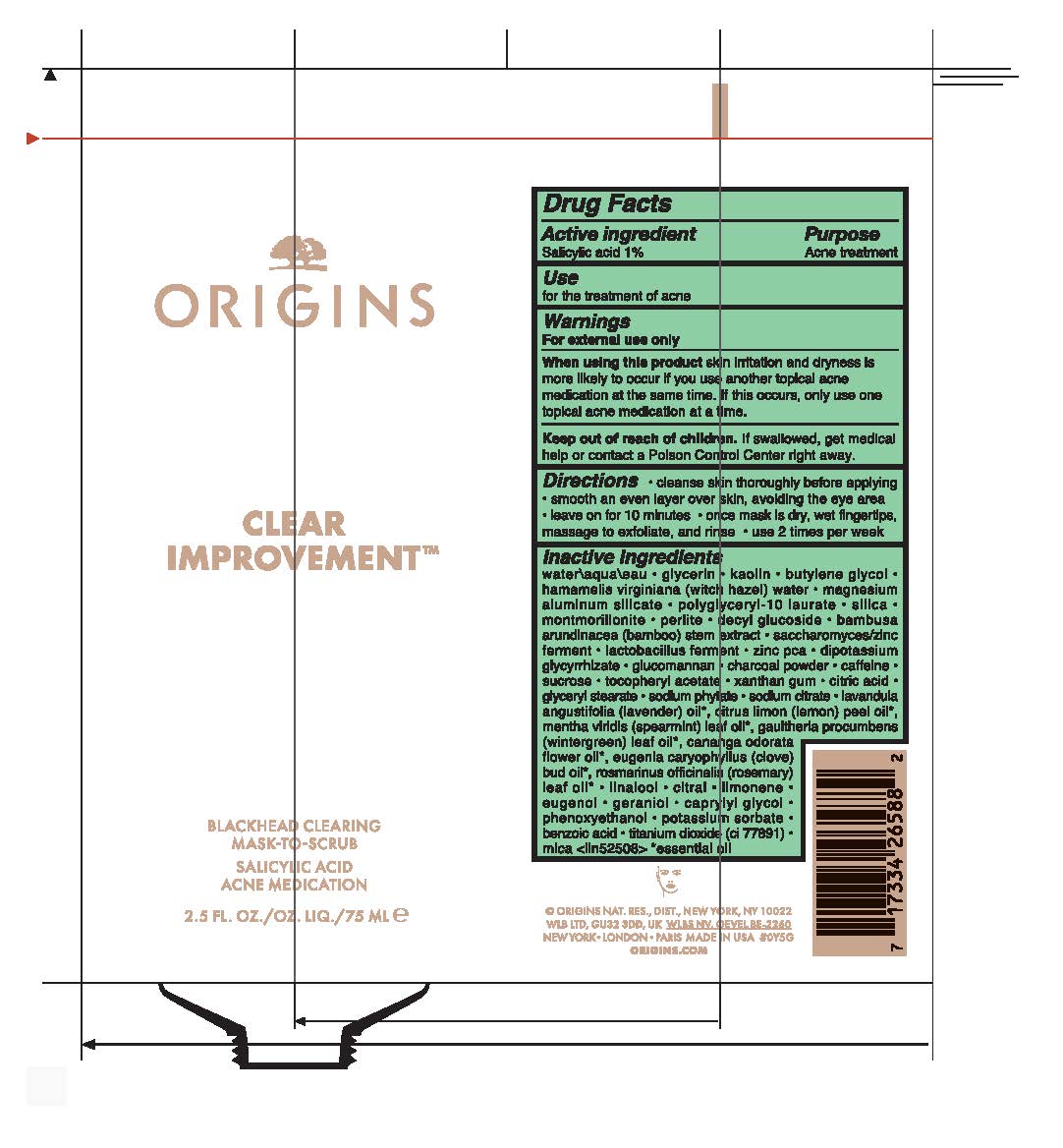
Label: CLEAR IMPROVEMENT BLACKHEAD CLEARING MASK TO SCRUB- salicylic acid liquid
- NDC Code(s): 59427-118-01, 59427-118-02
- Packager: ORIGINS NATURAL RESOURCES INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
-
Inactive Ingredients
water\aqua\eau,glycerin,kaolin,butylene glycol,hamamelis virginiana (witch hazel) water,magnesium aluminum silicate,polyglyceryl-10 laurate,silica,montmorillonite,perlite,decyl glucoside,bambusa arundinacea (bamboo) stem extract,saccharomyces/zinc ferment,lactobacillus ferment,zinc pca,dipotassium glycyrrhizate,glucomannan,charcoal powder,caffeine,sucrose,tocopheryl acetate,xanthan gum,citric acid,glyceryl stearate,sodium phytate,sodium citrate,lavandula angustifolia (lavender) oil*, citrus limon (lemon) peel oil*, mentha viridis (spearmint) leaf oil*, gaultheria procumbens (wintergreen) leaf oil*, cananga odorata flower oil*, eugenia caryophyllus (clove) bud oil*, rosmarinus officinalis (rosemary) leaf oil*,linalool,citral,limonene,eugenol,geraniol,caprylyl glycol,phenoxyethanol,potassium sorbate,benzoic acid,titanium dioxide (ci 77891),mica <iln52508>
* essential oil - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLEAR IMPROVEMENT BLACKHEAD CLEARING MASK TO SCRUB
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59427-118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CANANGA OIL (UNII: 8YOY78GNNX) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MICA (UNII: V8A1AW0880) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SPEARMINT OIL (UNII: C3M81465G5) CITRAL (UNII: T7EU0O9VPP) METHYL SALICYLATE (UNII: LAV5U5022Y) SODIUM CITRATE (UNII: 1Q73Q2JULR) MONTMORILLONITE (UNII: A585MN1H2L) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ZINC PIDOLATE (UNII: C32PQ86DH4) LAVENDER OIL (UNII: ZBP1YXW0H8) LIMONENE, (+)- (UNII: GFD7C86Q1W) GERANIOL (UNII: L837108USY) GLYCERIN (UNII: PDC6A3C0OX) KAOLIN (UNII: 24H4NWX5CO) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) POLYGLYCERYL-10 LAURATE (UNII: MPJ2Q8WI8G) BAMBUSA BAMBOS STEM (UNII: NRA4497HC5) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) XANTHAN GUM (UNII: TTV12P4NEE) EUGENOL (UNII: 3T8H1794QW) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PERLITE (UNII: 0SG101ZGK9) CAFFEINE (UNII: 3G6A5W338E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) LAUROYL LYSINE (UNII: 113171Q70B) LEMON OIL (UNII: I9GRO824LL) ROSEMARY OIL (UNII: 8LGU7VM393) LINALOOL, (+/-)- (UNII: D81QY6I88E) PHENOXYETHANOL (UNII: HIE492ZZ3T) KONJAC MANNAN (UNII: 36W3E5TAMG) WATER (UNII: 059QF0KO0R) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) CLOVE OIL (UNII: 578389D6D0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BENZOIC ACID (UNII: 8SKN0B0MIM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59427-118-01 75 mL in 1 TUBE; Type 0: Not a Combination Product 02/05/2024 2 NDC:59427-118-02 15 mL in 1 TUBE; Type 0: Not a Combination Product 02/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 02/05/2024 Labeler - ORIGINS NATURAL RESOURCES INC. (611716283) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(59427-118) , label(59427-118) , pack(59427-118)