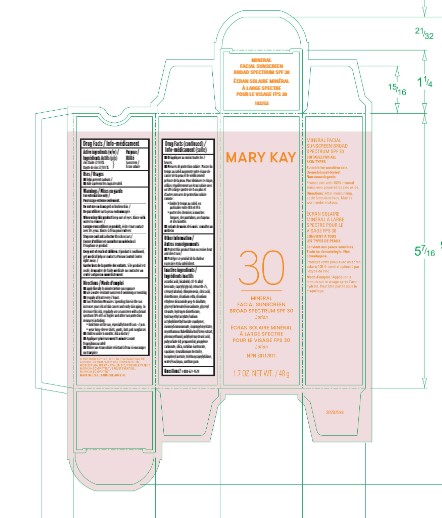
Label: MARY KAY MINERAL FACIAL SUNSCREEN BROAD SPECTRUM SPF30- zinc oxide lotion
- NDC Code(s): 51531-3253-7
- Packager: Mary Kay Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive ingredients
ascorbic acid, bisabolol, c12-15 alkyl
benzoate, caprylyl glycol, ceteareth-25,
cetearyl alcohol, chlorphenesin, citric acid,
dimethicone, disodium edta, disodium
ethylene dicocamide peg-15 disulfate,
glyceryl behenate/eicosadioate, glyceryl
stearate, hydrogen dimethicone,
hydroxyethyl acrylate/sodium
acryloyldimethyl taurate copolymer,
isononyl isononanoate, isopropyl myristate,
myrothamnus flabellifolia leaf/stem extract,
phenoxyethanol, polyhydroxystearic acid,
polysorbate 60, propanediol, propylene
carbonate, silica, sorbitan isostearate,
squalane, stearalkonium hectorite,
tocopheryl acetate, triethoxycaprylylsilane,
water/eau/aqua, xanthan gum. - Other information
- Questions or comments?
- Principal Display Panel - 1.7 oz
-
INGREDIENTS AND APPEARANCE
MARY KAY MINERAL FACIAL SUNSCREEN BROAD SPECTRUM SPF30
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51531-3253 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 22.973 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) ASCORBIC ACID (UNII: PQ6CK8PD0R) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) LEVOMENOL (UNII: 24WE03BX2T) CETEARETH-25 (UNII: 8FA93U5T67) SQUALANE (UNII: GW89575KF9) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) XANTHAN GUM (UNII: TTV12P4NEE) GLYCERYL BEHENATE/EICOSADIOATE (UNII: 73CJJ317SR) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) MYROTHAMNUS FLABELLIFOLIA LEAF (UNII: 6Y9E0R40J5) POLYSORBATE 60 (UNII: CAL22UVI4M) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PROPANEDIOL (UNII: 5965N8W85T) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51531-3253-7 1 in 1 CARTON 02/24/2024 1 48 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/24/2024 Labeler - Mary Kay Inc. (049994452) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 103978839 manufacture(51531-3253)