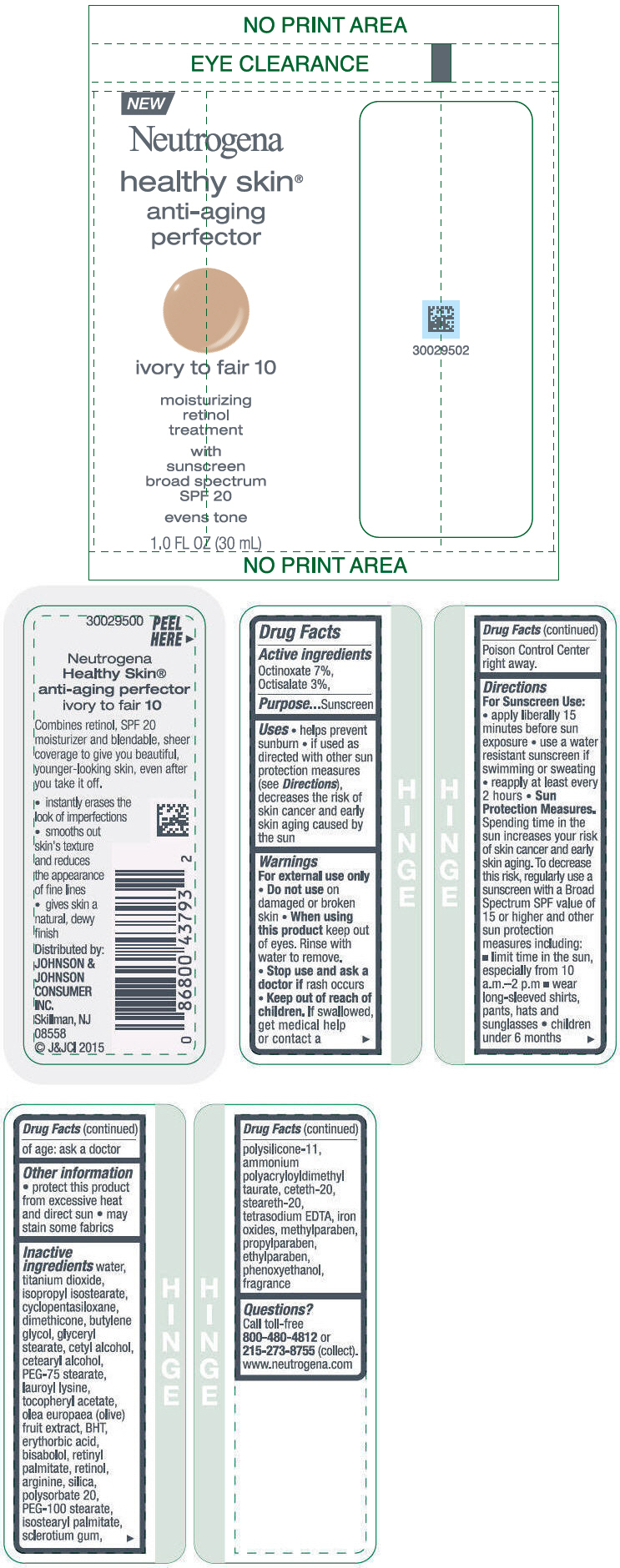
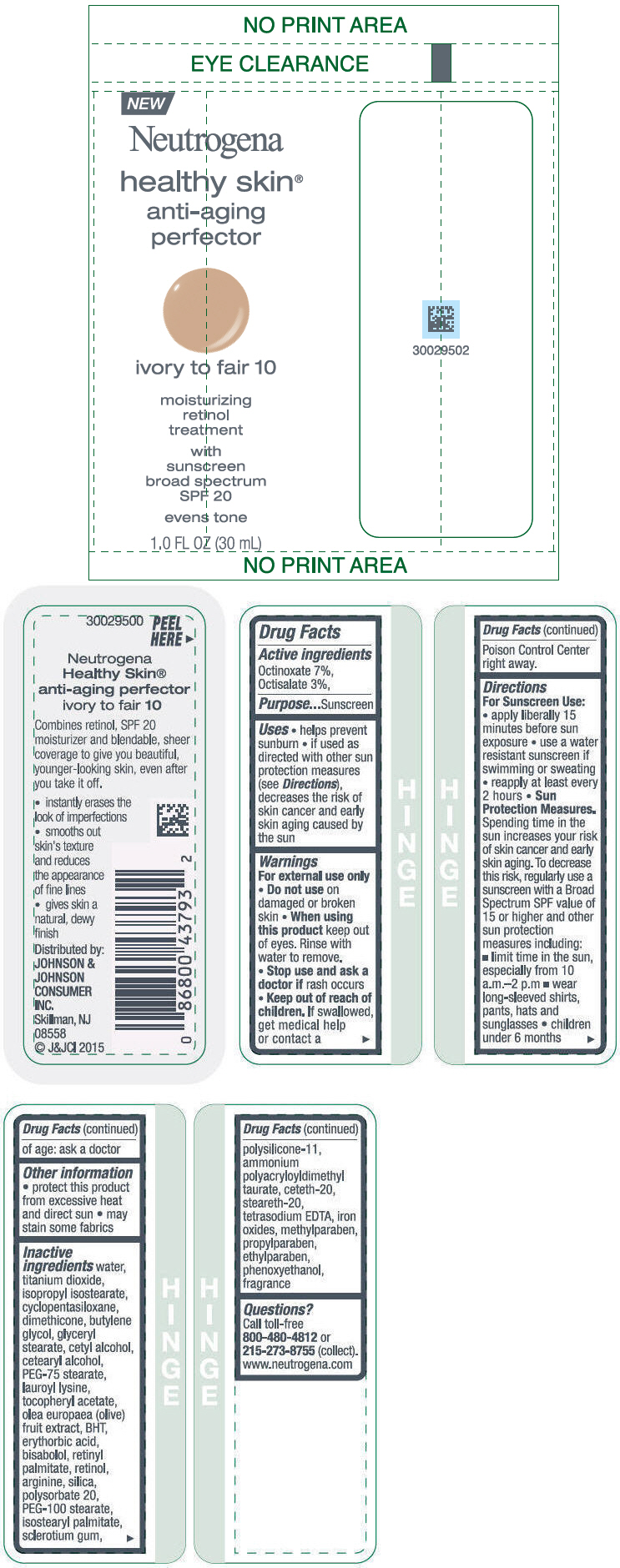
Label: NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 IVORY TO FAIR 10- octinoxate and octisalate lotion
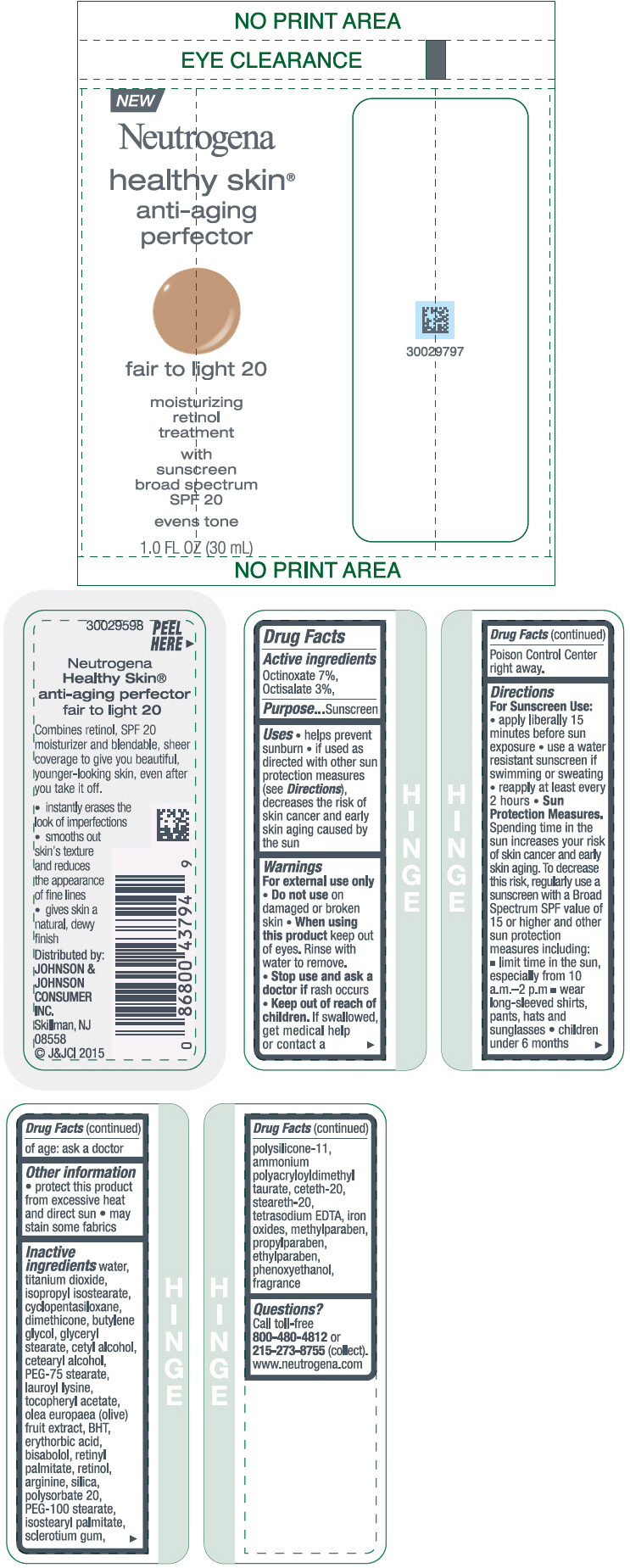
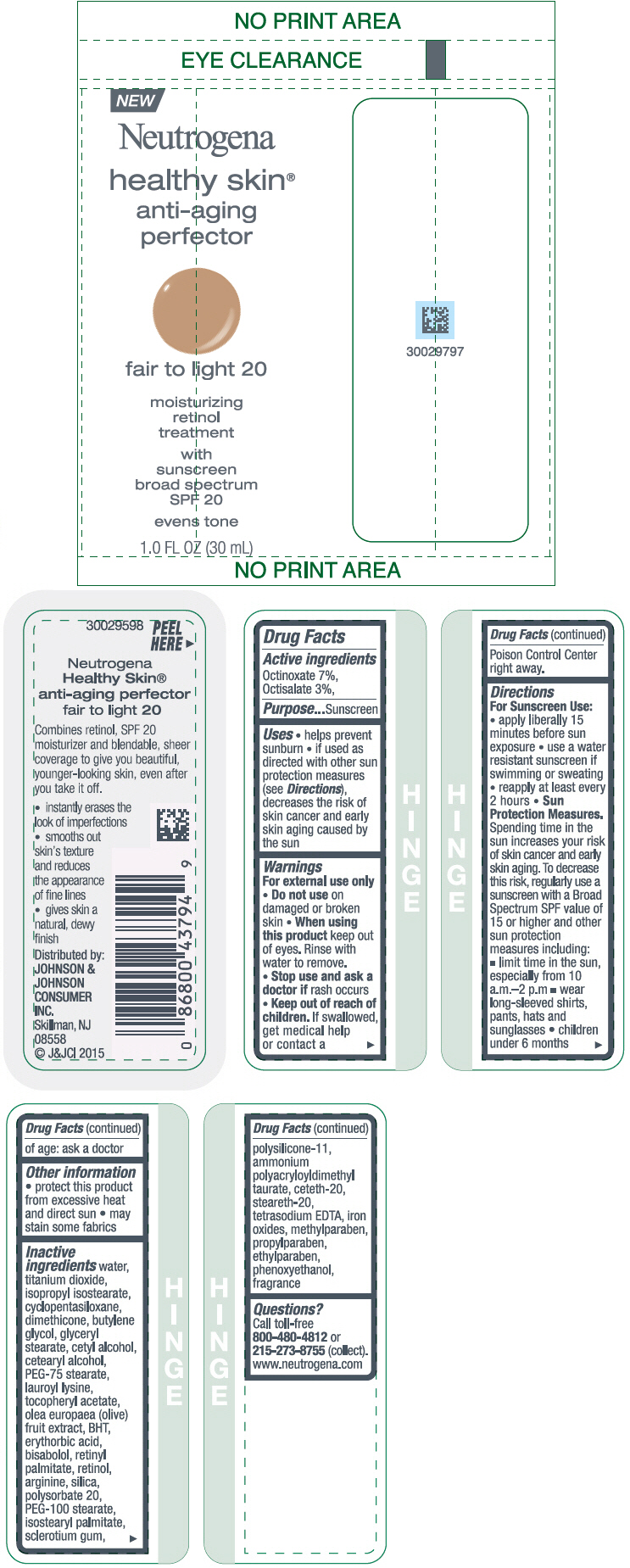
NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 FAIR TO LIGHT 20- octinoxate and octisalate lotion
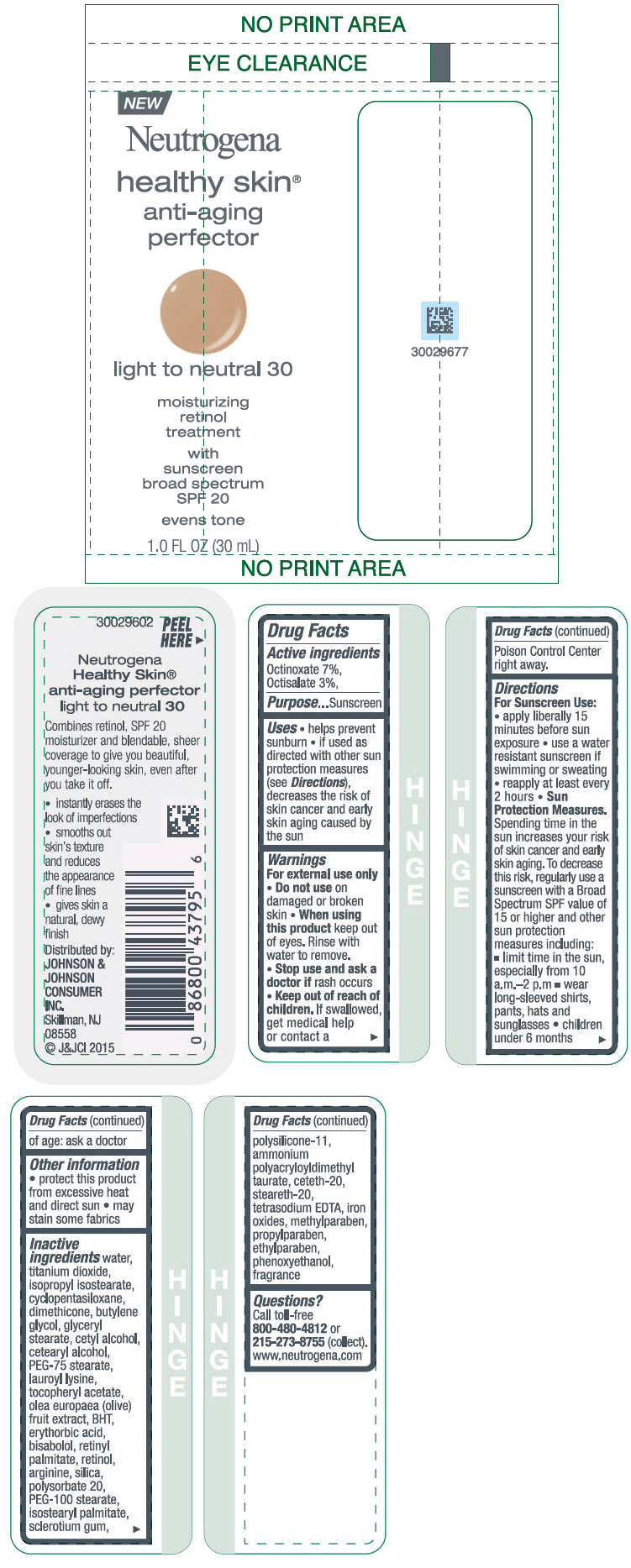
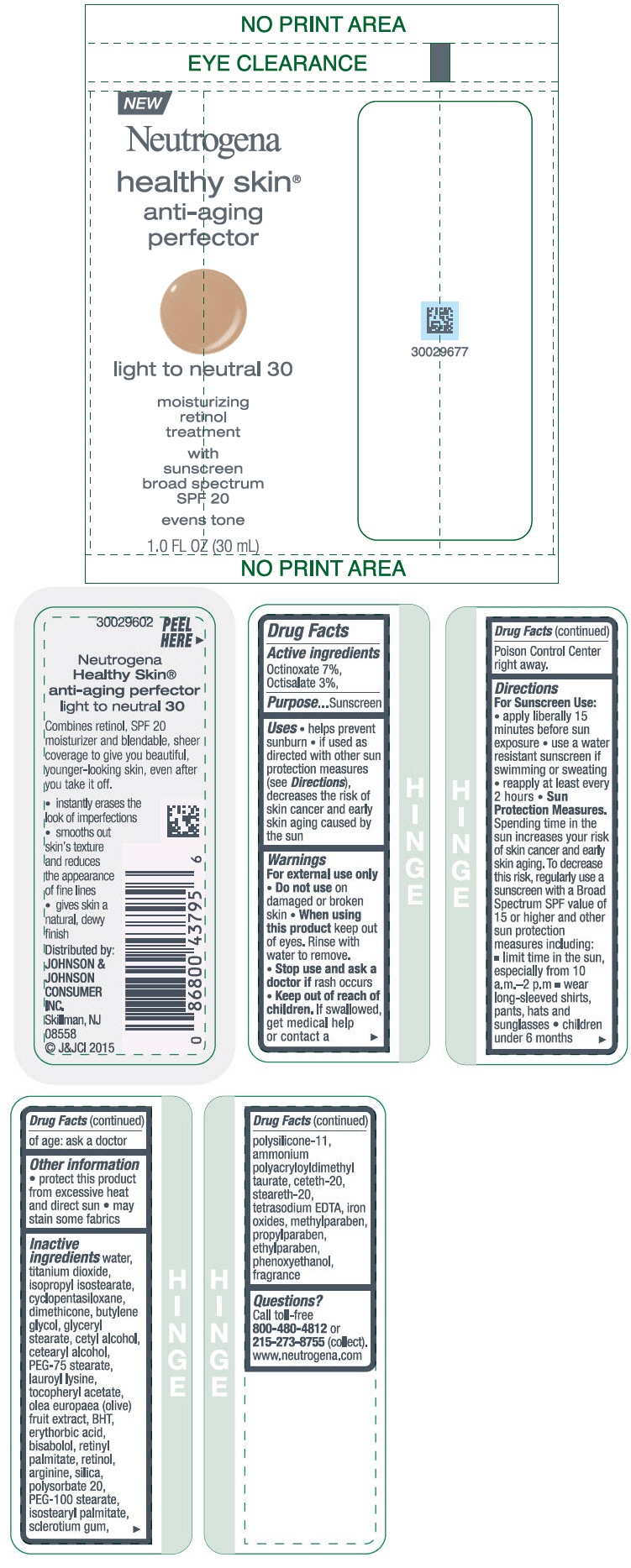
NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 LIGHT TO NEUTRAL 30- octinoxate and octisalate lotion
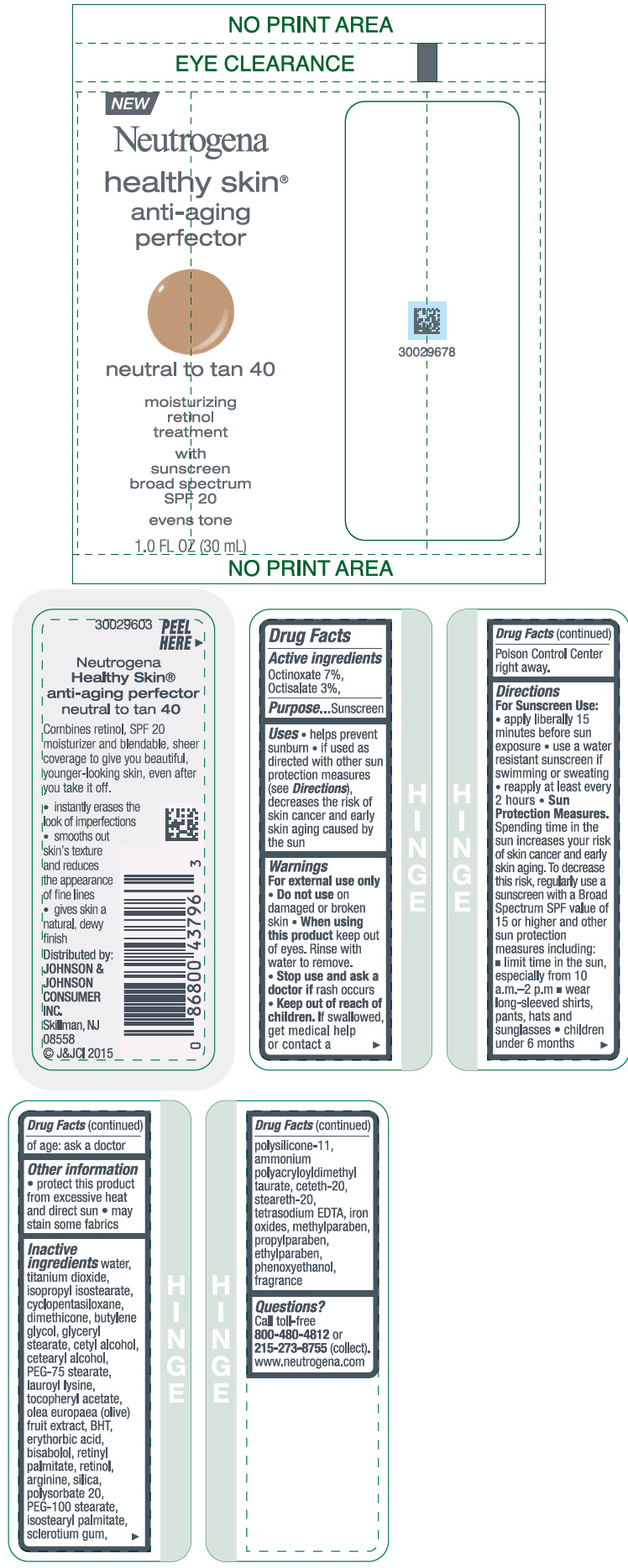
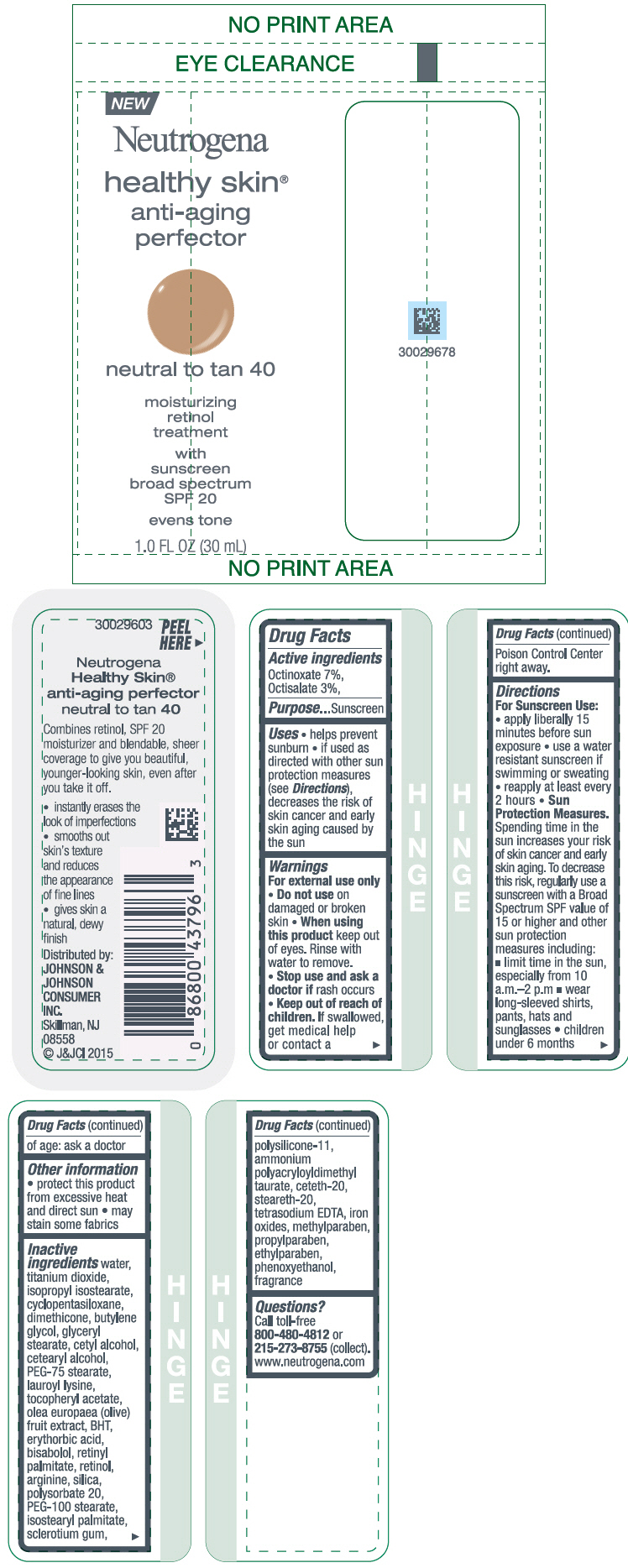
NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 NEUTRAL TO TAN 40- octinoxate and octisalate lotion
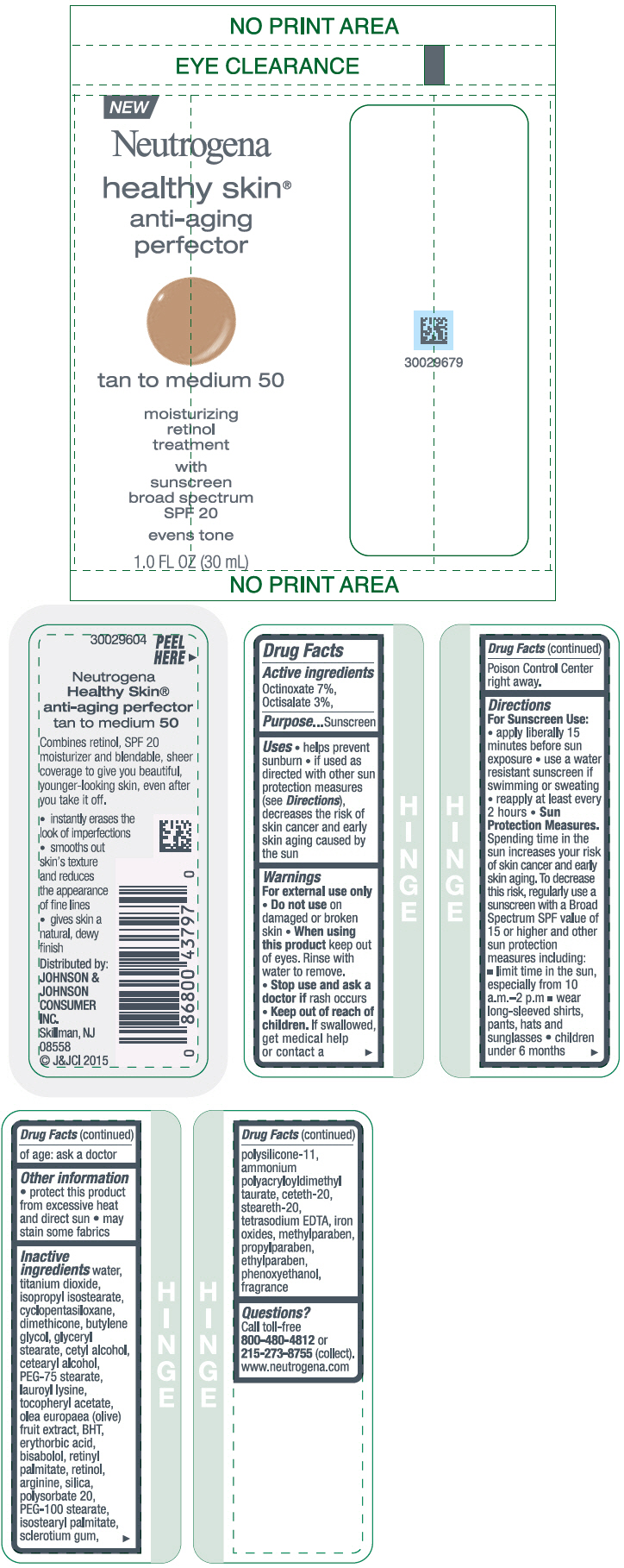
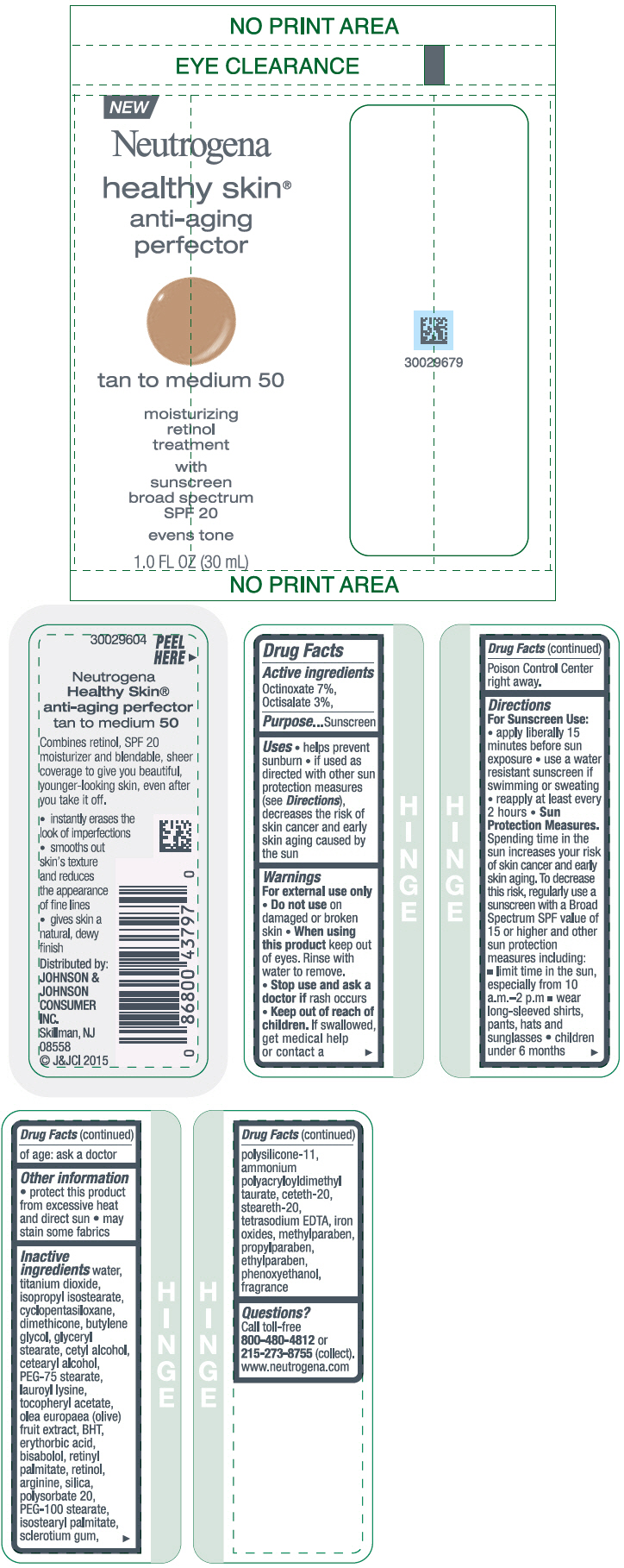
NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 TAN TO MEDIUM 50- octinoxate and octisalate lotion
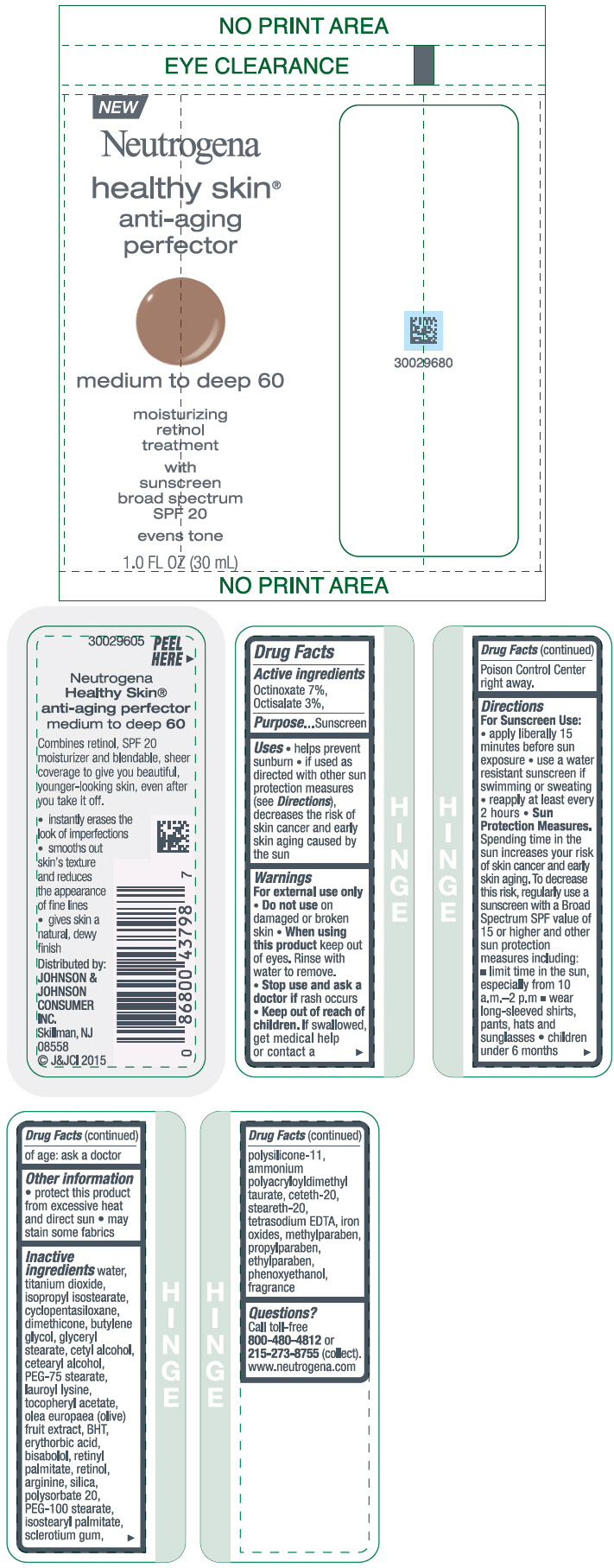
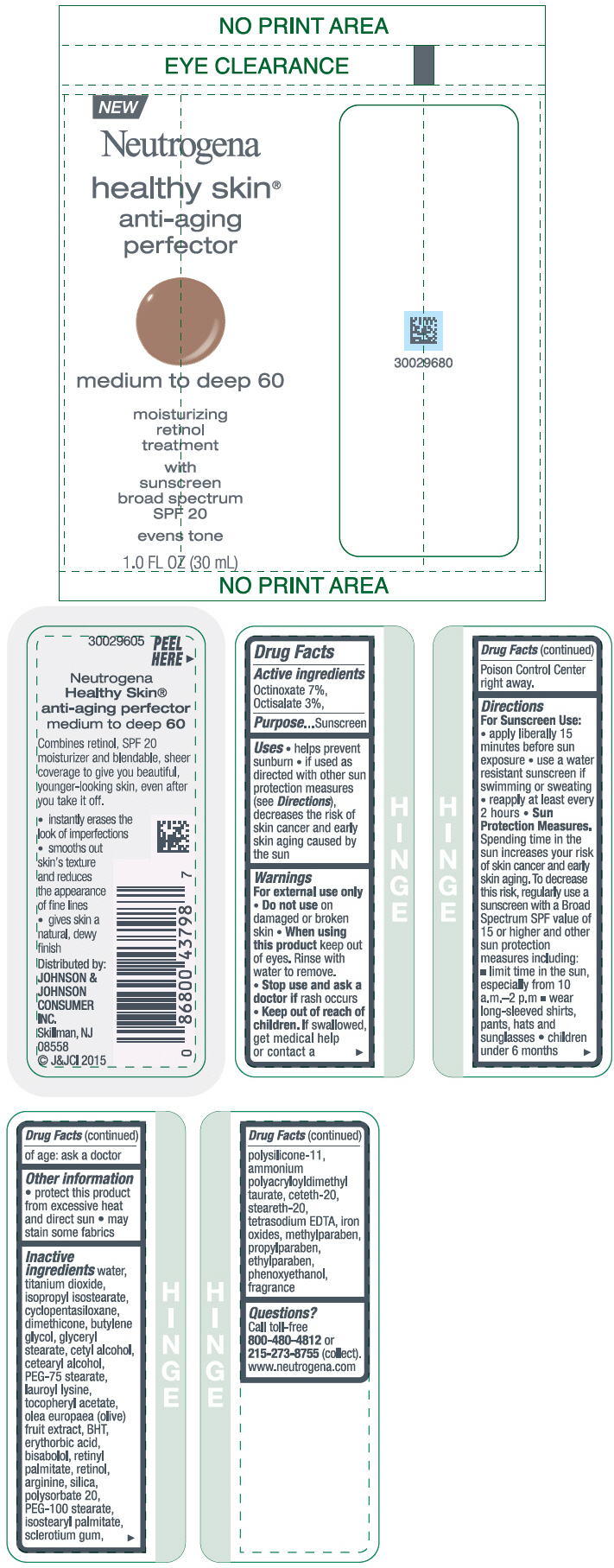
NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 MEDIUM TO DEEP 60- octinoxate and octisalate lotion
-
NDC Code(s):
69968-0076-1,
69968-0077-1,
69968-0078-1,
69968-0079-1, view more69968-0080-1, 69968-0081-1
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
- Other Information
-
Inactive Ingredients
Water, Titanium Dioxide, Isopropyl Isostearate, Cyclopentasiloxane, Dimethicone, Butylene Glycol, Glyceryl Stearate, Cetyl Alcohol, Cetearyl Alcohol, PEG-75 Stearate, Lauroyl Lysine, Tocopheryl Acetate, Olea Europaea (Olive) Fruit Extract, BHT, Erythorbic Acid, Bisabolol, Retinyl Palmitate, Retinol, Arginine, Slica, Polysorbate 20, PEG-100 Stearate, Isostearyl Palmitate, Sclerotium Gum, Polysilicone-11, Ammonium Polyacryloyldimethyl Taurate, Ceteth-20, Steareth-20, Tetrasodium EDTA, Iron Oxides, Methylparaben, Propylparaben, Ethylparaben, Phenoxyethanol, Fragrance
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - ivory to fair 10
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - fair to light 20
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - light to neutral 30
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - neutral to tan 40
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - tan to medium 50
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - medium to deep 60
-
INGREDIENTS AND APPEARANCE
NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 IVORY TO FAIR 10
octinoxate and octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0076 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 70 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ISOPROPYL ISOSTEARATE (UNII: C67IXB9Y7T) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-75 STEARATE (UNII: OT38R0N74H) LAUROYL LYSINE (UNII: 113171Q70B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BLACK OLIVE (UNII: 2M6QWV94OC) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ERYTHORBIC ACID (UNII: 311332OII1) LEVOMENOL (UNII: 24WE03BX2T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) RETINOL (UNII: G2SH0XKK91) ARGININE (UNII: 94ZLA3W45F) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYSORBATE 20 (UNII: 7T1F30V5YH) PEG-100 STEARATE (UNII: YD01N1999R) ISOSTEARYL PALMITATE (UNII: 9EHU0R7ER1) BETASIZOFIRAN (UNII: 2X51AD1X3T) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) EDETATE SODIUM (UNII: MP1J8420LU) FERRIC OXIDE RED (UNII: 1K09F3G675) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ETHYLPARABEN (UNII: 14255EXE39) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0076-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2015 NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 FAIR TO LIGHT 20
octinoxate and octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0077 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 70 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength PEG-100 STEARATE (UNII: YD01N1999R) ISOSTEARYL PALMITATE (UNII: 9EHU0R7ER1) BETASIZOFIRAN (UNII: 2X51AD1X3T) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) EDETATE SODIUM (UNII: MP1J8420LU) FERRIC OXIDE RED (UNII: 1K09F3G675) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ETHYLPARABEN (UNII: 14255EXE39) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ISOPROPYL ISOSTEARATE (UNII: C67IXB9Y7T) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-75 STEARATE (UNII: OT38R0N74H) LAUROYL LYSINE (UNII: 113171Q70B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BLACK OLIVE (UNII: 2M6QWV94OC) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ERYTHORBIC ACID (UNII: 311332OII1) LEVOMENOL (UNII: 24WE03BX2T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) RETINOL (UNII: G2SH0XKK91) ARGININE (UNII: 94ZLA3W45F) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYSORBATE 20 (UNII: 7T1F30V5YH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0077-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2015 NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 LIGHT TO NEUTRAL 30
octinoxate and octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0078 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 70 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ISOPROPYL ISOSTEARATE (UNII: C67IXB9Y7T) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-75 STEARATE (UNII: OT38R0N74H) LAUROYL LYSINE (UNII: 113171Q70B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BLACK OLIVE (UNII: 2M6QWV94OC) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ERYTHORBIC ACID (UNII: 311332OII1) LEVOMENOL (UNII: 24WE03BX2T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) RETINOL (UNII: G2SH0XKK91) ARGININE (UNII: 94ZLA3W45F) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYSORBATE 20 (UNII: 7T1F30V5YH) PEG-100 STEARATE (UNII: YD01N1999R) ISOSTEARYL PALMITATE (UNII: 9EHU0R7ER1) BETASIZOFIRAN (UNII: 2X51AD1X3T) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) EDETATE SODIUM (UNII: MP1J8420LU) FERRIC OXIDE RED (UNII: 1K09F3G675) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ETHYLPARABEN (UNII: 14255EXE39) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0078-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2015 NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 NEUTRAL TO TAN 40
octinoxate and octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0079 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 70 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ISOPROPYL ISOSTEARATE (UNII: C67IXB9Y7T) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-75 STEARATE (UNII: OT38R0N74H) LAUROYL LYSINE (UNII: 113171Q70B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BLACK OLIVE (UNII: 2M6QWV94OC) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ERYTHORBIC ACID (UNII: 311332OII1) LEVOMENOL (UNII: 24WE03BX2T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) RETINOL (UNII: G2SH0XKK91) ARGININE (UNII: 94ZLA3W45F) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYSORBATE 20 (UNII: 7T1F30V5YH) PEG-100 STEARATE (UNII: YD01N1999R) ISOSTEARYL PALMITATE (UNII: 9EHU0R7ER1) BETASIZOFIRAN (UNII: 2X51AD1X3T) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) EDETATE SODIUM (UNII: MP1J8420LU) FERRIC OXIDE RED (UNII: 1K09F3G675) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ETHYLPARABEN (UNII: 14255EXE39) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0079-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2015 NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 TAN TO MEDIUM 50
octinoxate and octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0080 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 70 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ISOSTEARATE (UNII: C67IXB9Y7T) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-75 STEARATE (UNII: OT38R0N74H) LAUROYL LYSINE (UNII: 113171Q70B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BLACK OLIVE (UNII: 2M6QWV94OC) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ERYTHORBIC ACID (UNII: 311332OII1) LEVOMENOL (UNII: 24WE03BX2T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) RETINOL (UNII: G2SH0XKK91) ARGININE (UNII: 94ZLA3W45F) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYSORBATE 20 (UNII: 7T1F30V5YH) PEG-100 STEARATE (UNII: YD01N1999R) ISOSTEARYL PALMITATE (UNII: 9EHU0R7ER1) BETASIZOFIRAN (UNII: 2X51AD1X3T) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) EDETATE SODIUM (UNII: MP1J8420LU) FERRIC OXIDE RED (UNII: 1K09F3G675) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ETHYLPARABEN (UNII: 14255EXE39) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0080-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2015 NEUTROGENA HEALTHY SKIN ANTI-AGING PERFECTOR SUNSCREEN BROAD SPECTRUM SPF 20 MEDIUM TO DEEP 60
octinoxate and octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0081 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 70 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ISOPROPYL ISOSTEARATE (UNII: C67IXB9Y7T) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-75 STEARATE (UNII: OT38R0N74H) LAUROYL LYSINE (UNII: 113171Q70B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BLACK OLIVE (UNII: 2M6QWV94OC) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ERYTHORBIC ACID (UNII: 311332OII1) LEVOMENOL (UNII: 24WE03BX2T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) RETINOL (UNII: G2SH0XKK91) ARGININE (UNII: 94ZLA3W45F) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYSORBATE 20 (UNII: 7T1F30V5YH) PEG-100 STEARATE (UNII: YD01N1999R) ISOSTEARYL PALMITATE (UNII: 9EHU0R7ER1) BETASIZOFIRAN (UNII: 2X51AD1X3T) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) EDETATE SODIUM (UNII: MP1J8420LU) FERRIC OXIDE RED (UNII: 1K09F3G675) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ETHYLPARABEN (UNII: 14255EXE39) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0081-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2015 Labeler - Johnson & Johnson Consumer Inc. (118772437)