Label: HAND SANITIZER FOAM- ethanol alcohol aerosol, foam
- NDC Code(s): 83584-225-01, 83584-225-02, 83584-225-03
- Packager: PurCel Labs LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- WHEN USING
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
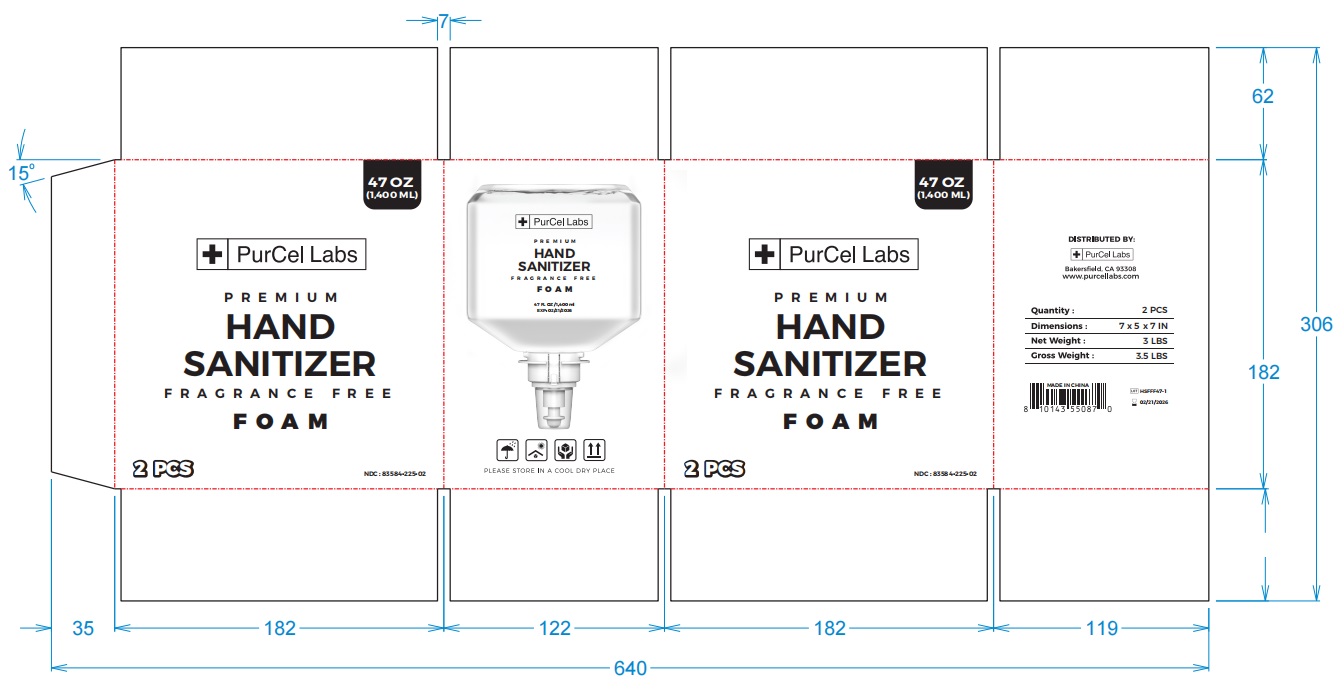
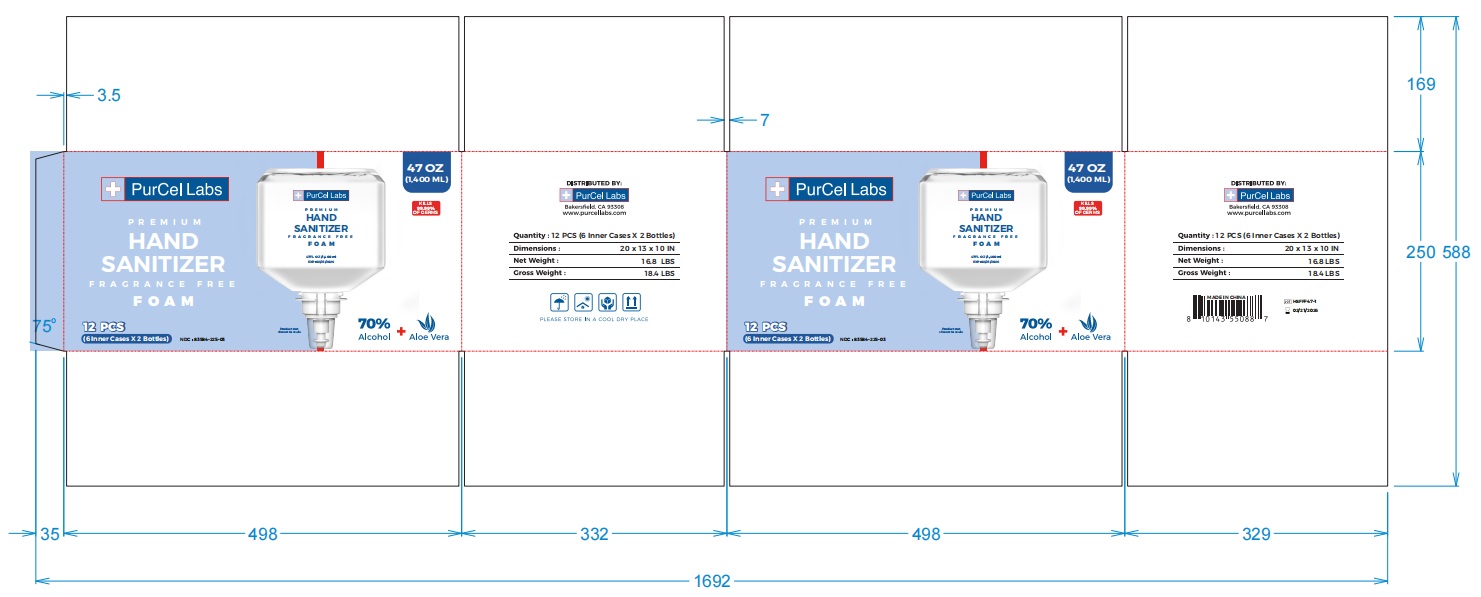
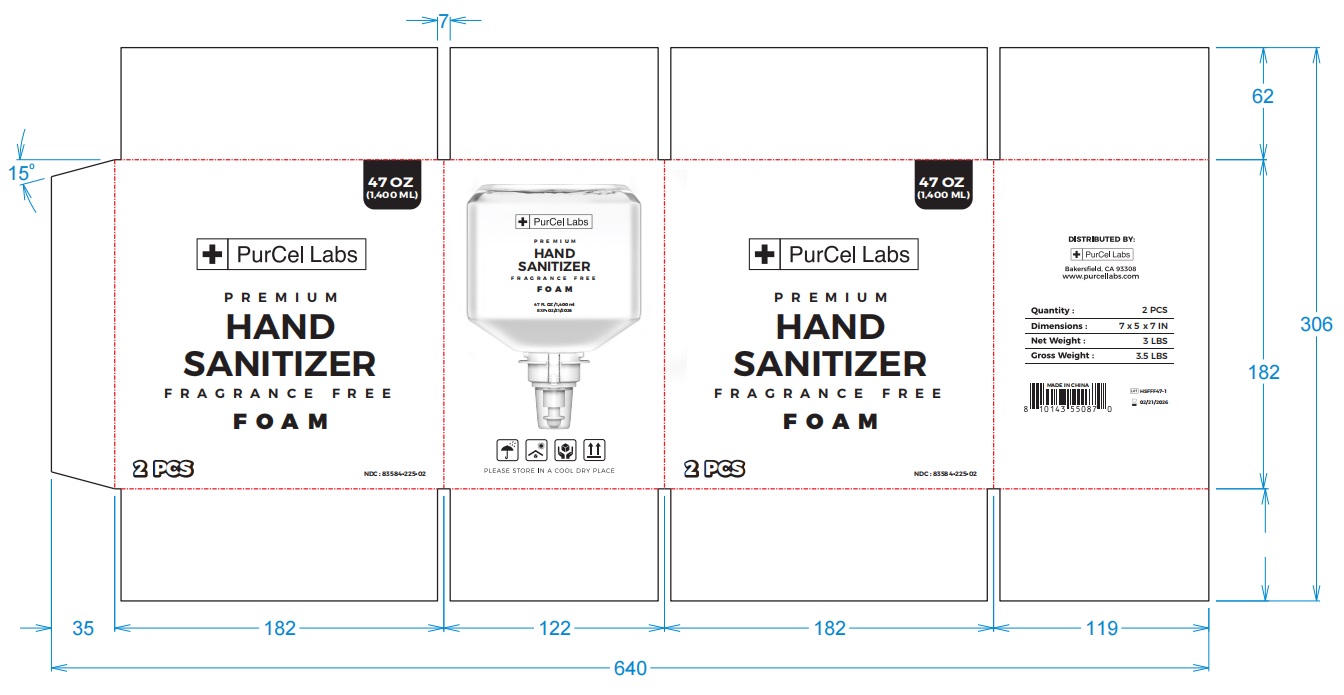
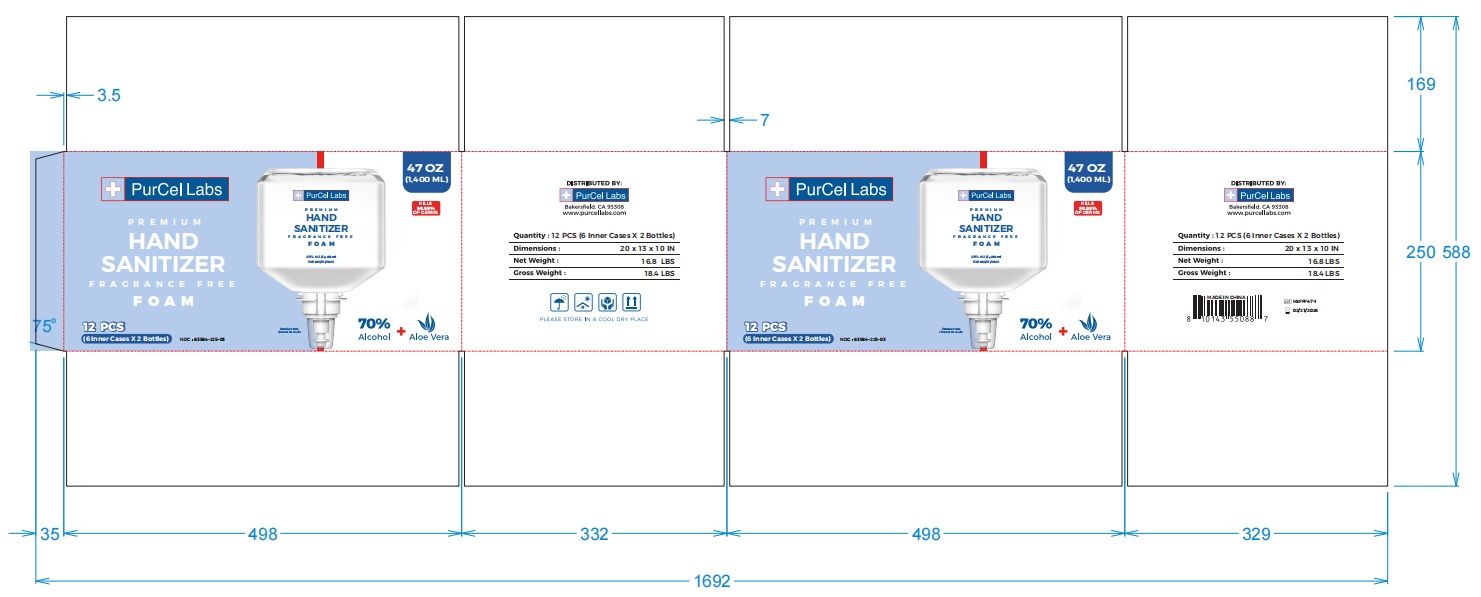
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HAND SANITIZER FOAM
ethanol alcohol aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83584-225 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) ALOE VERA LEAF (UNII: ZY81Z83H0X) PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) GLYCERIN (UNII: PDC6A3C0OX) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83584-225-03 6 in 1 CARTON 02/29/2024 1 NDC:83584-225-02 2 in 1 BOX 1 NDC:83584-225-01 1400 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 02/29/2024 Labeler - PurCel Labs LLC (127672531) Registrant - PurCel Labs LLC (127672531) Establishment Name Address ID/FEI Business Operations Zhongshan Dermey Commodity Co.,Ltd 550280464 manufacture(83584-225)