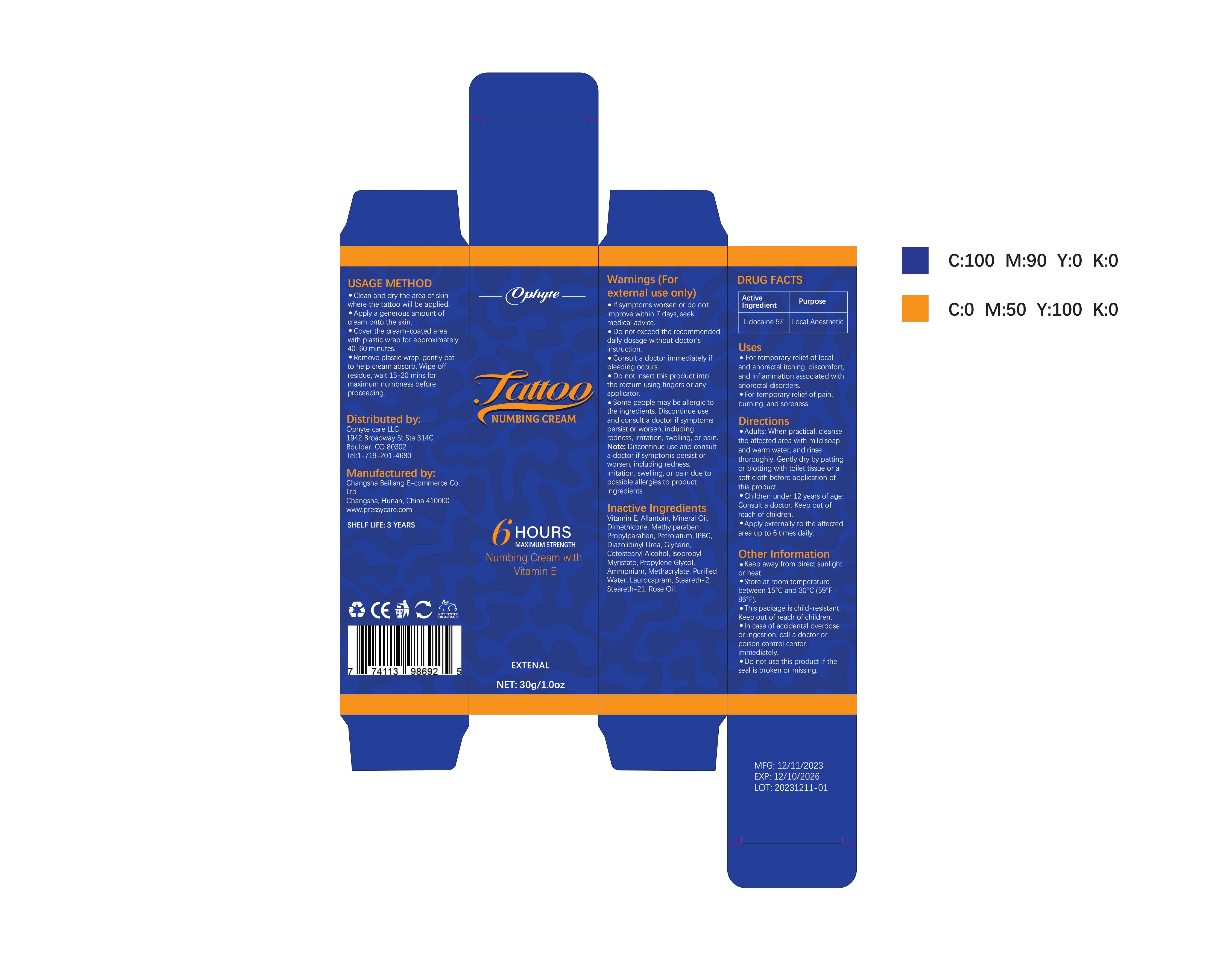
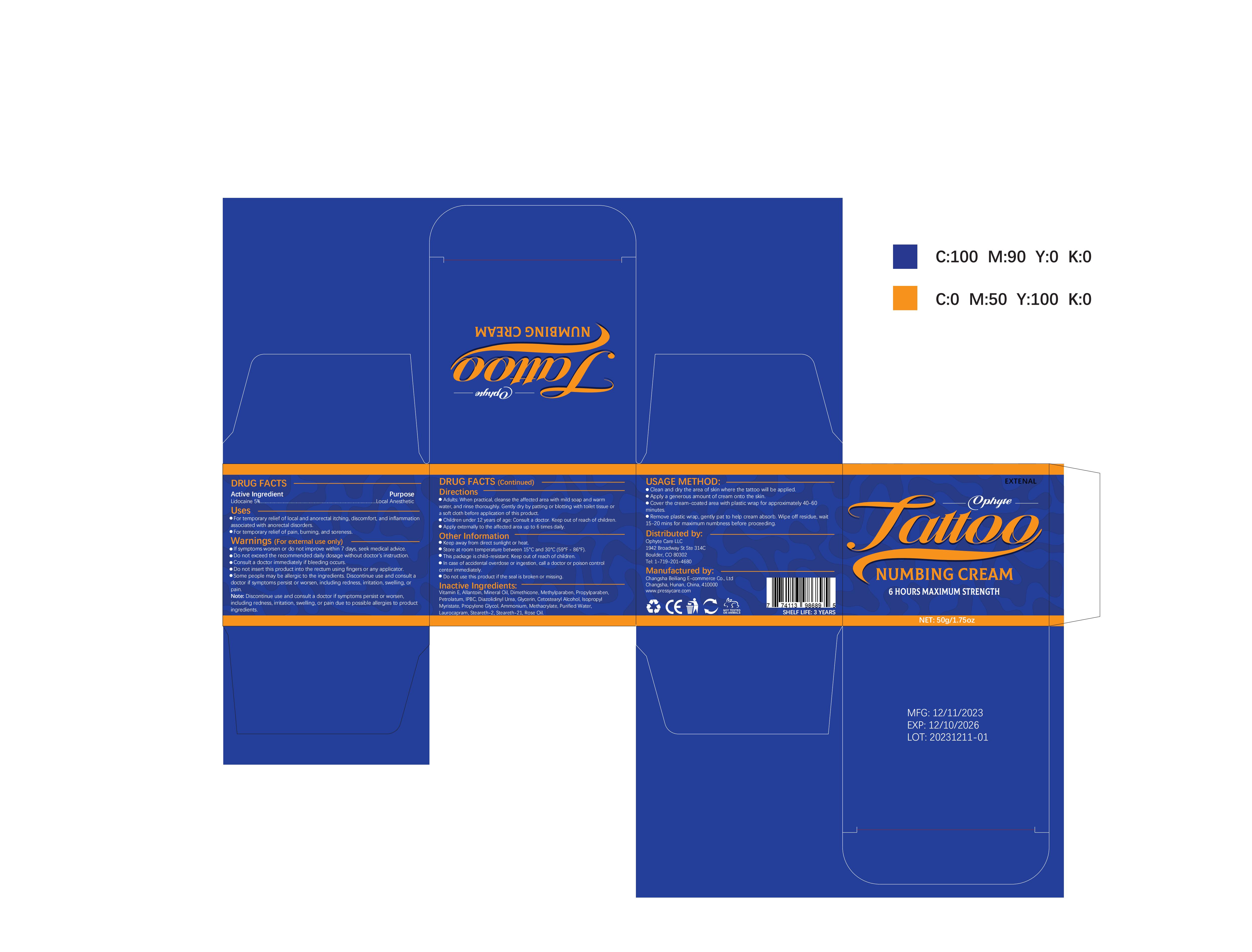
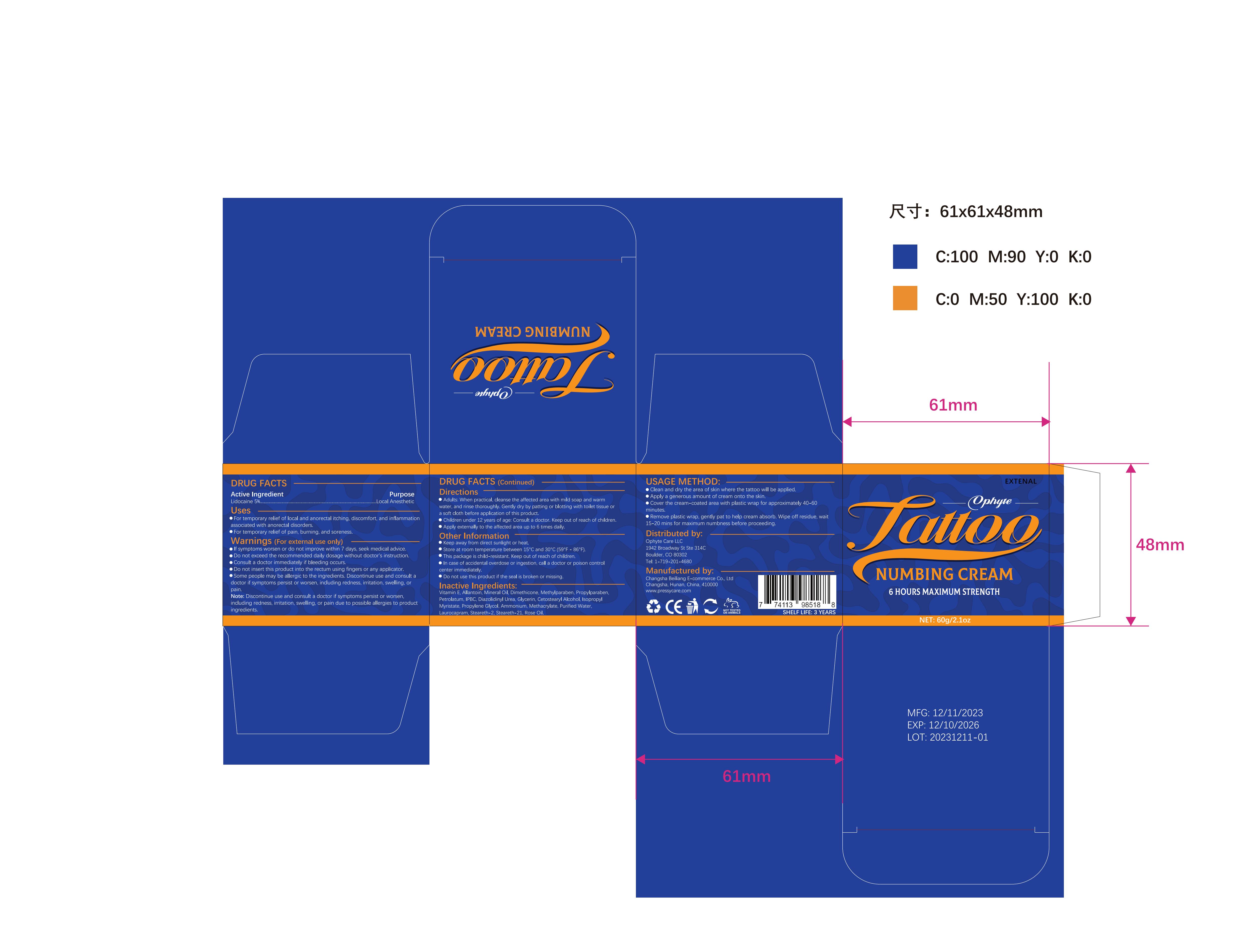
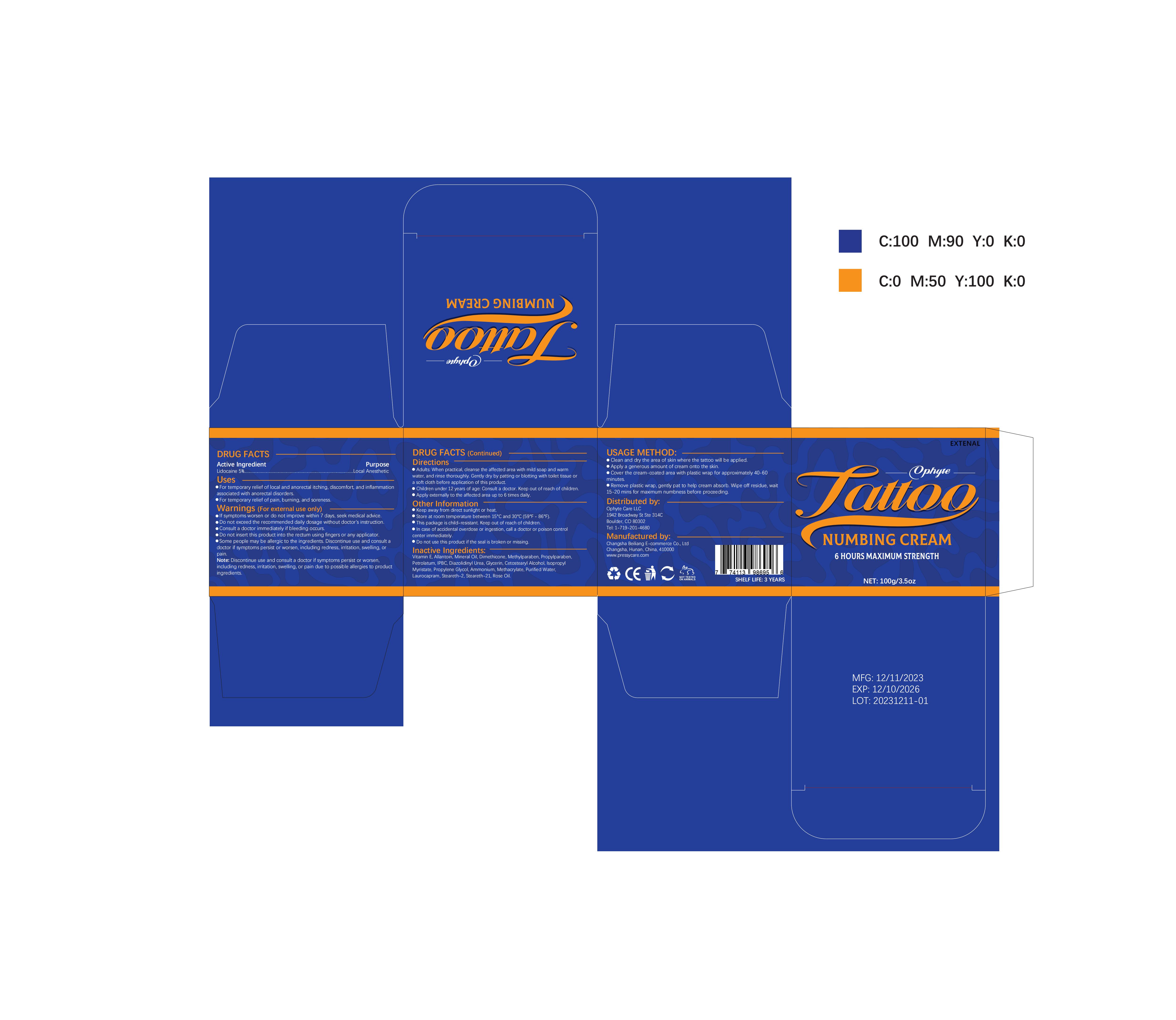
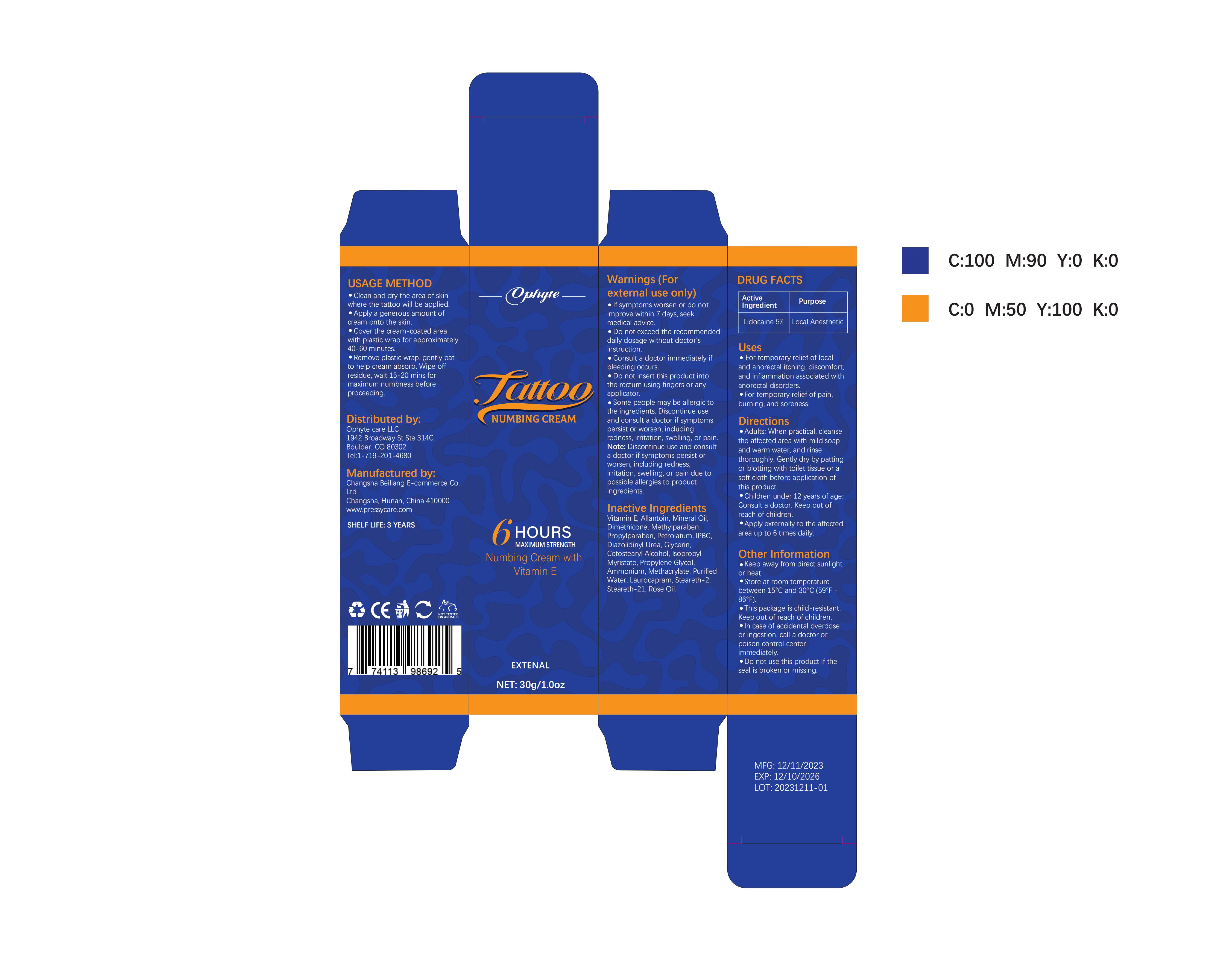
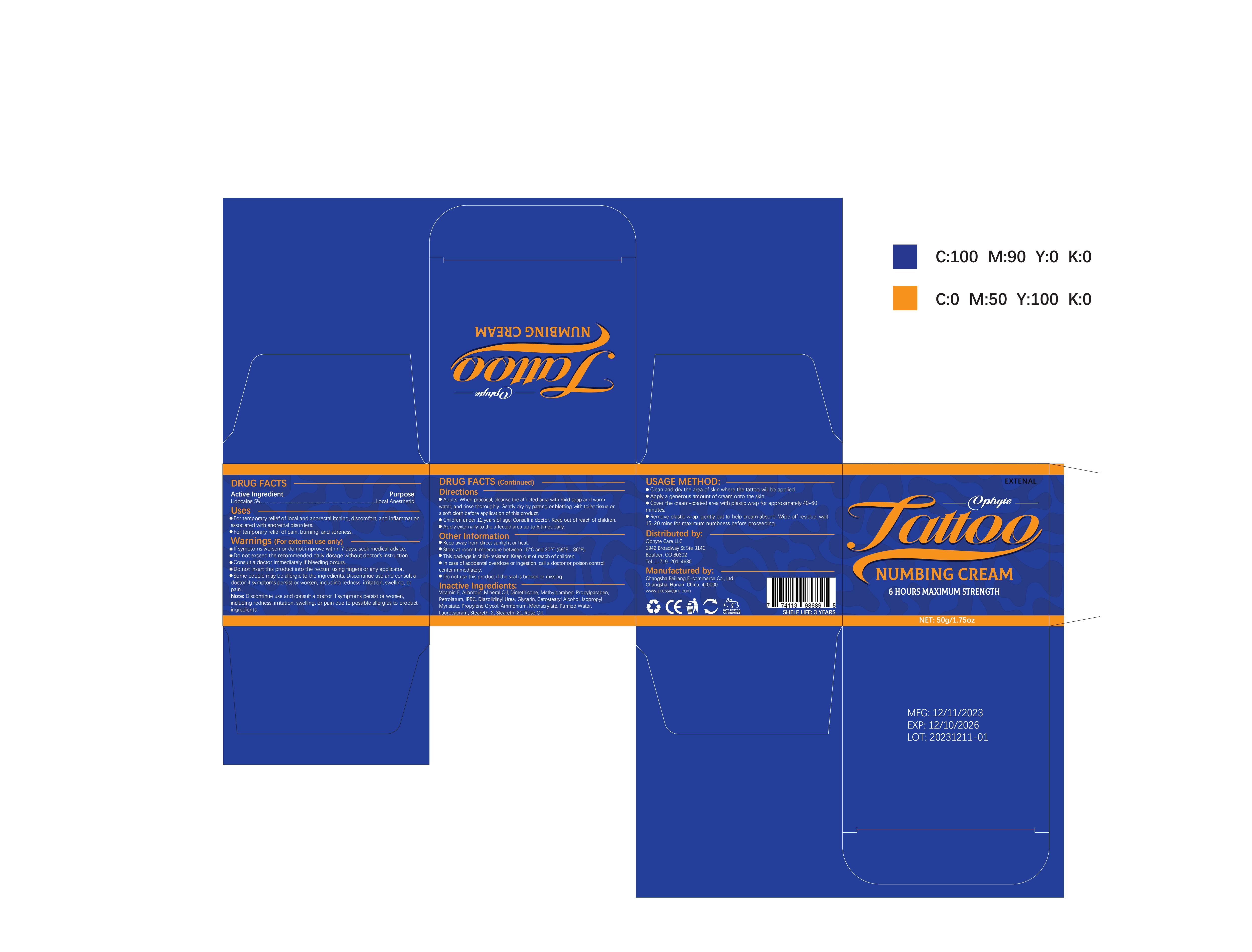
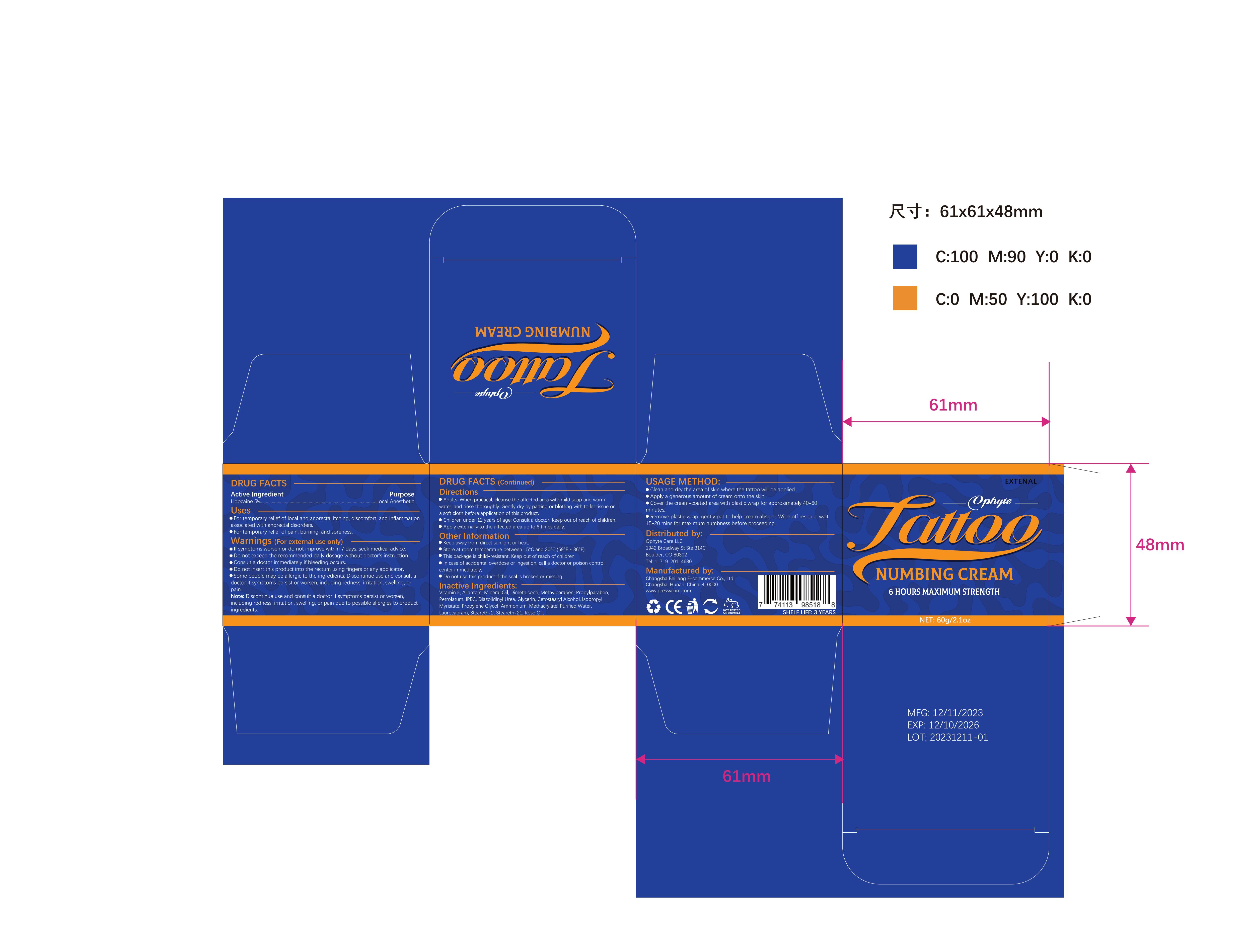
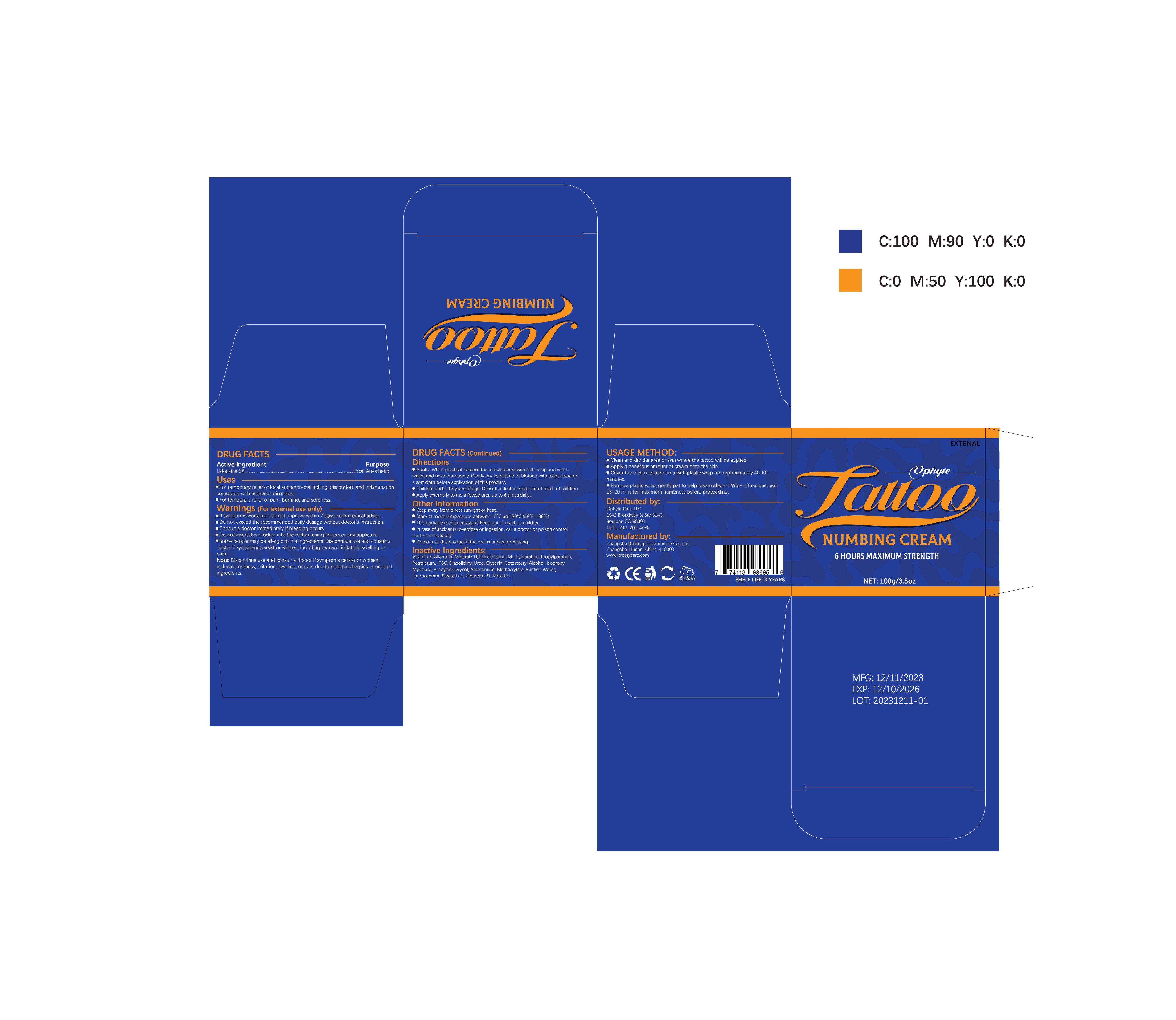
Label: OPHYTE TATTOO NUMBING CREAM- numbing cream cream
- NDC Code(s): 83887-002-01, 83887-002-02, 83887-002-03, 83887-002-04
- Packager: Changsha Beiliang E-commerce Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
-
Warnings
lf symptoms worsen or do notimprove within 7 days, seek medicaadvice.
Do not exceed the recommendeodaily dosage without doctor's
instruction.
Consult a doctor immediately if bleeding occurs.
Do not insert this product into the rectum using fingers or any applicator.
Some people may be allergic to the ingredients.Discontinue use and consult a doctor if symptoms persist or worsen,including redness,
irritation, swelling, or pain.
Note: Discontinue use and consult a
doctor if symptoms persist or
worsen, including redness, irritation.
swelling, or pain due to possible
allergies to product ingredients - Do not use
- When Using
-
When Using
If symptoms worsen or do not improve within 7 days, seek medicaladvice.Do not exceed the recommended daily dosage without doctor'sinstruction.Consult a doctor immediately if bleeding occurs.
Do not insert this product into the rectum using fingers or any applicator.
Some people may be allergic to the ingredients. Discontinue use and consult a doctor if symptoms persistor worsen,including redness,irritation,swelling,or pain. - Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
-
Other information
Keep away from direct sunlight orheat.
Store at room temperaturebetween 15°C and 30°C(59°F-86°F).
This package is child-resistant.Keep out of reach of children. In case of accidental overdose oringestion, call a doctor or poisoncontrol center immediately. Do not use this product if the sealis broken or missing. - Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OPHYTE TATTOO NUMBING CREAM
numbing cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83887-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 5 g in 100 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) ALLANTOIN (UNII: 344S277G0Z) GLYCERIN (UNII: PDC6A3C0OX) ROSE OIL (UNII: WUB68Y35M7) DIMETHICONE (UNII: 92RU3N3Y1O) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) METHYLPARABEN (UNII: A2I8C7HI9T) AMMONIUM METHACRYLATE (UNII: J2243103QO) STEARETH-2 (UNII: V56DFE46J5) MINERAL OIL (UNII: T5L8T28FGP) PROPYLPARABEN (UNII: Z8IX2SC1OH) PETROLATUM (UNII: 4T6H12BN9U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAUROCAPRAM (UNII: 1F3X9DRV9X) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) WATER (UNII: 059QF0KO0R) STEARETH-21 (UNII: 53J3F32P58) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83887-002-01 30 g in 1 TUBE; Type 0: Not a Combination Product 12/22/2023 2 NDC:83887-002-02 50 g in 1 BOTTLE; Type 0: Not a Combination Product 12/22/2023 3 NDC:83887-002-03 60 g in 1 BOTTLE; Type 0: Not a Combination Product 12/22/2023 4 NDC:83887-002-04 100 g in 1 BOTTLE; Type 0: Not a Combination Product 12/22/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/22/2023 Labeler - Changsha Beiliang E-commerce Co., Ltd (632138945) Establishment Name Address ID/FEI Business Operations Changsha Beiliang E-commerce Co., Ltd 632138945 label(83887-002) , manufacture(83887-002)