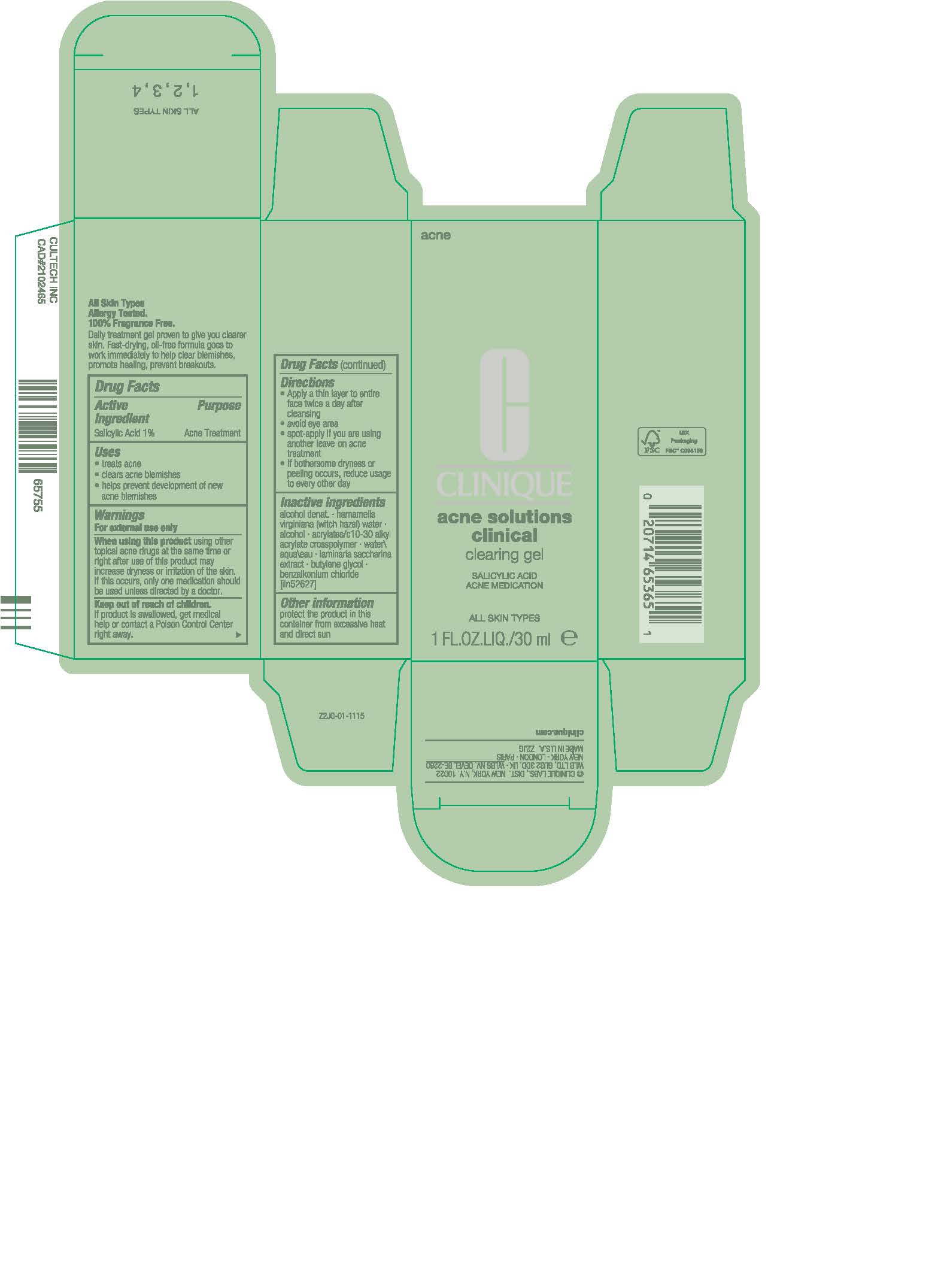
Label: ACNE SOLUTIONS CLINICAL CLEARING GEL- salicylic acid gel
- NDC Code(s): 49527-116-01, 49527-116-02, 49527-116-03
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Other information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACNE SOLUTIONS CLINICAL CLEARING GEL
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-116 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) WITCH HAZEL (UNII: 101I4J0U34) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) PSEUDOPTEROGORGIA ELISABETHAE (UNII: UDY3H1OUX5) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-116-01 1 in 1 CARTON 12/21/2023 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:49527-116-02 1 in 1 CARTON 12/21/2023 2 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:49527-116-03 1 in 1 CARTON 12/21/2023 3 3 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/21/2023 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder N.V. 370151326 manufacture(49527-116) , pack(49527-116) , label(49527-116) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(49527-116) , pack(49527-116) , label(49527-116) Establishment Name Address ID/FEI Business Operations NORTHTEC KEYSTONE 949264774 pack(49527-116) , label(49527-116) Establishment Name Address ID/FEI Business Operations Northtec LLC 943871157 pack(49527-116) , label(49527-116) Establishment Name Address ID/FEI Business Operations PALC 078364654 pack(49527-116) , label(49527-116)