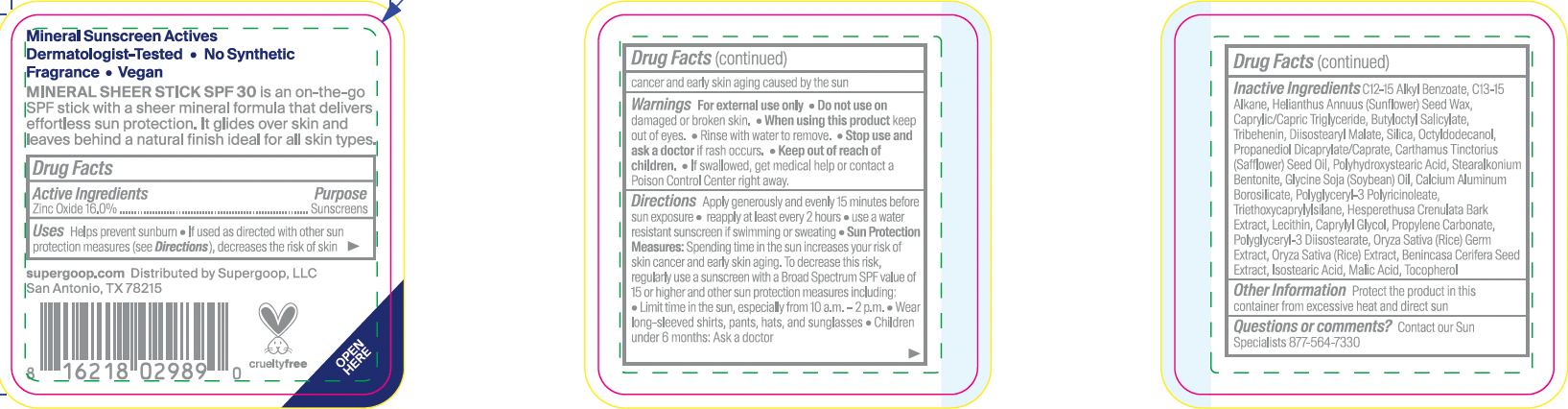
Label: MINERAL SHEER STICK- zinc oxide stick
- NDC Code(s): 75936-637-01
- Packager: Supergoop, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Directions
Apply generously and evenly 15 minutes before sun exposure
- Reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.- 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
-
INACTIVE INGREDIENT
Inactive Ingredients: C12-15 Alkyl Benzoate, C13-15 Alkane, Helianthus Annuus (Sunflower) Seed Wax,Caprylic/Capric Triglyceride, Butyloctyl Salicylate, Tribehenin, Diisostearyl Malate, Silica,Octyldodecanol, Propanediol Dicaprylate/Caprate, Carthamus Tinctorius (Safflower)SeedOil, Polyhydroxystearic Acid, Stearalkonium Bentonite, Glycine Soja(Soybean) Oil, CalciumAluminum Borosilicate, Polyglyceryl-3 Polyricinoleate, Triethoxycaprylylsilane,Hesperethusa Crenulata Bark Extract, Lecithin, Caprylyl Glycol, Propylene Carbonate,Polyglyceryl-3 Diisostearate, Oryza Sativa (Rice) Germ Extract, Oryza Sativa (Rice) Extract,Benincasa Cerifera Seed Extract, Isostearic Acid, Malic Acid, Tocopherol
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINERAL SHEER STICK
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-637 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 16 g in 100 g Inactive Ingredients Ingredient Name Strength NARINGI CRENULATA WHOLE (UNII: 3G168G2S4C) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) DISTEARYL METHYL BENZYL AMMONIUM CHLORIDE (UNII: CA3B7HQ34L) ISOSTEARIC ACID (UNII: X33R8U0062) SAFFLOWER OIL (UNII: 65UEH262IS) SOYBEAN OIL (UNII: 241ATL177A) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) PROPYLENE CARBONATE (UNII: 8D08K3S51E) RICE GERM (UNII: 7N2B70SFEZ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) C13-15 ALKANE (UNII: 114P5I43UJ) TRIBEHENIN (UNII: 8OC9U7TQZ0) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OCTYLDODECANOL (UNII: 461N1O614Y) PROPANEDIOL DICAPRYLATE/CAPRATE (UNII: F53961BX4F) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) BENINCASA HISPIDA SEED (UNII: 0EFG08DK6K) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-637-01 35 g in 1 CYLINDER; Type 0: Not a Combination Product 12/13/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/13/2023 Labeler - Supergoop, LLC (117061743) Registrant - Supergoop, LLC (117061743)