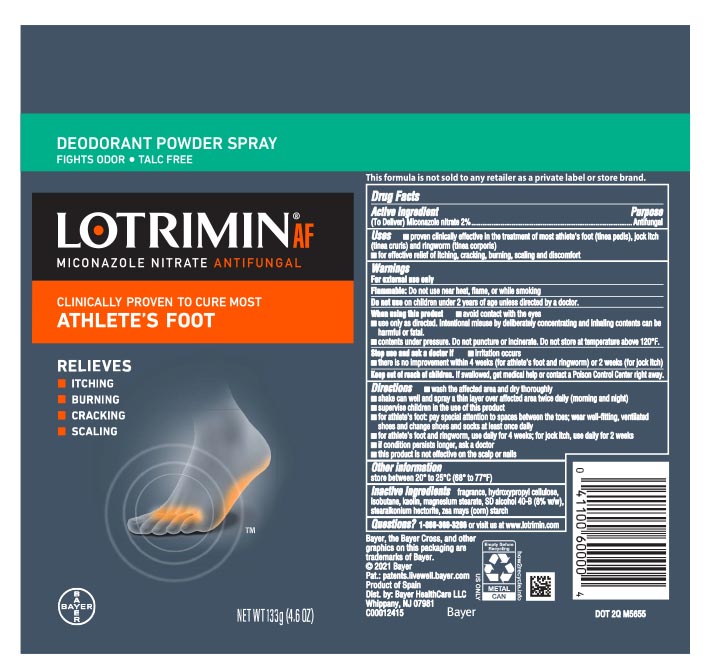
Label: LOTRIMIN AF DEODORANT- miconazole nitrate aerosol, powder
- NDC Code(s): 11523-0135-1
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Flammable
For external use only
flammable: Do not use near heat, flame, or while smoking
Do not use on children under 2 years of age unless directed by a doctor
When using this product
- avoid contact with the eyes
- use only as directed. Intentional misuse by deliberately concentrating and inhaling contents can be harmful or fatal.
- contents under pressure. Do not puncture or incinerate. Do not store at temperature above 120˚F.
-
Directions
- wash affected area and dry thoroughly
- shake can well and spray a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- for athlete's foot: pay special attention to spaces between the toes; wear well-fitting, ventilated shoes and change shoes and socks at least once daily
- for athlete's foot and ringworm, use daily for 4 weeks; for jock itch, use daily for 2 weeks
- if condition persists longer, ask a doctor
- this product is not effective on the scalp or nails
- Other information
- Questions?
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 133 g Can Label
-
INGREDIENTS AND APPEARANCE
LOTRIMIN AF DEODORANT
miconazole nitrate aerosol, powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-0135 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 20 mg in 1 g Inactive Ingredients Ingredient Name Strength STEARALKONIUM HECTORITE (UNII: OLX698AH5P) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) KAOLIN (UNII: 24H4NWX5CO) ISOBUTANE (UNII: BXR49TP611) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (White to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-0135-1 133 g in 1 CAN; Type 0: Not a Combination Product 11/29/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 11/29/2023 Labeler - Bayer HealthCare LLC. (112117283)