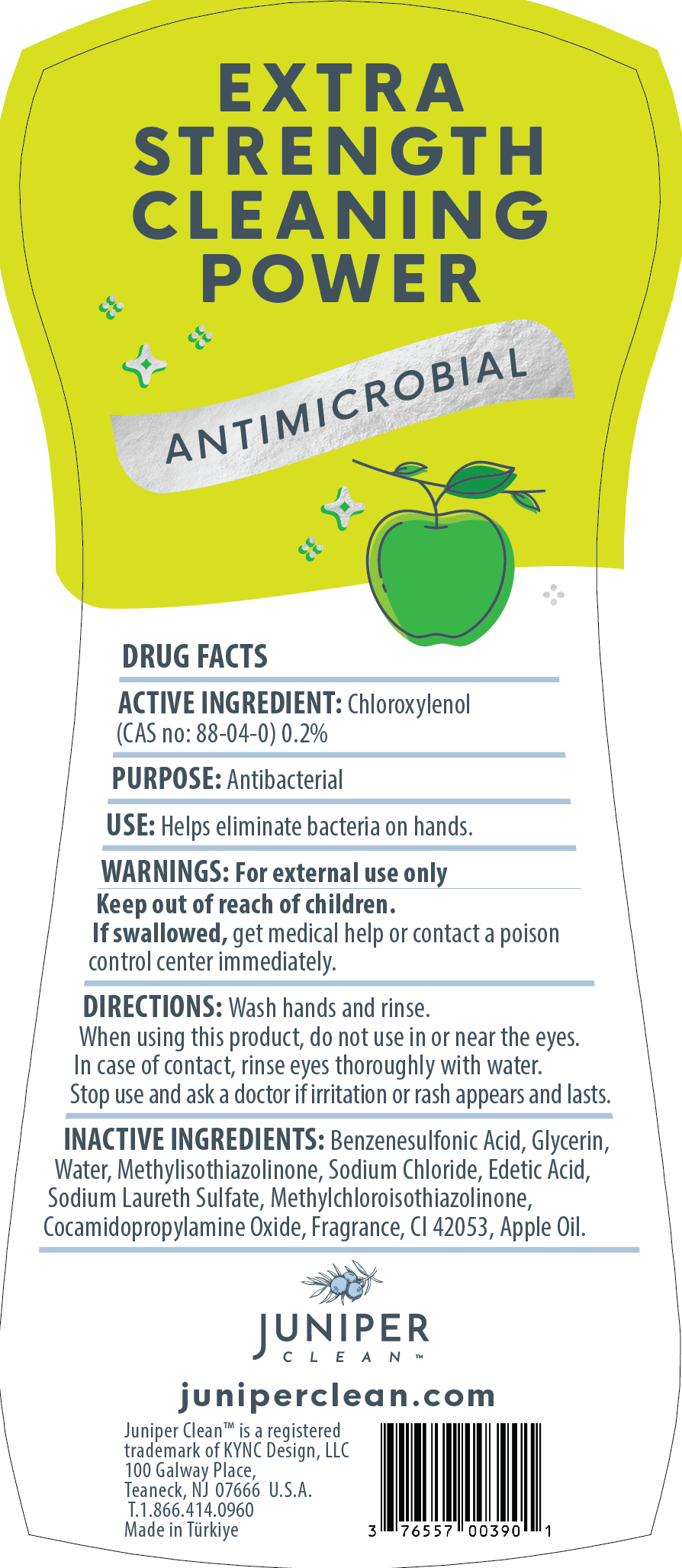
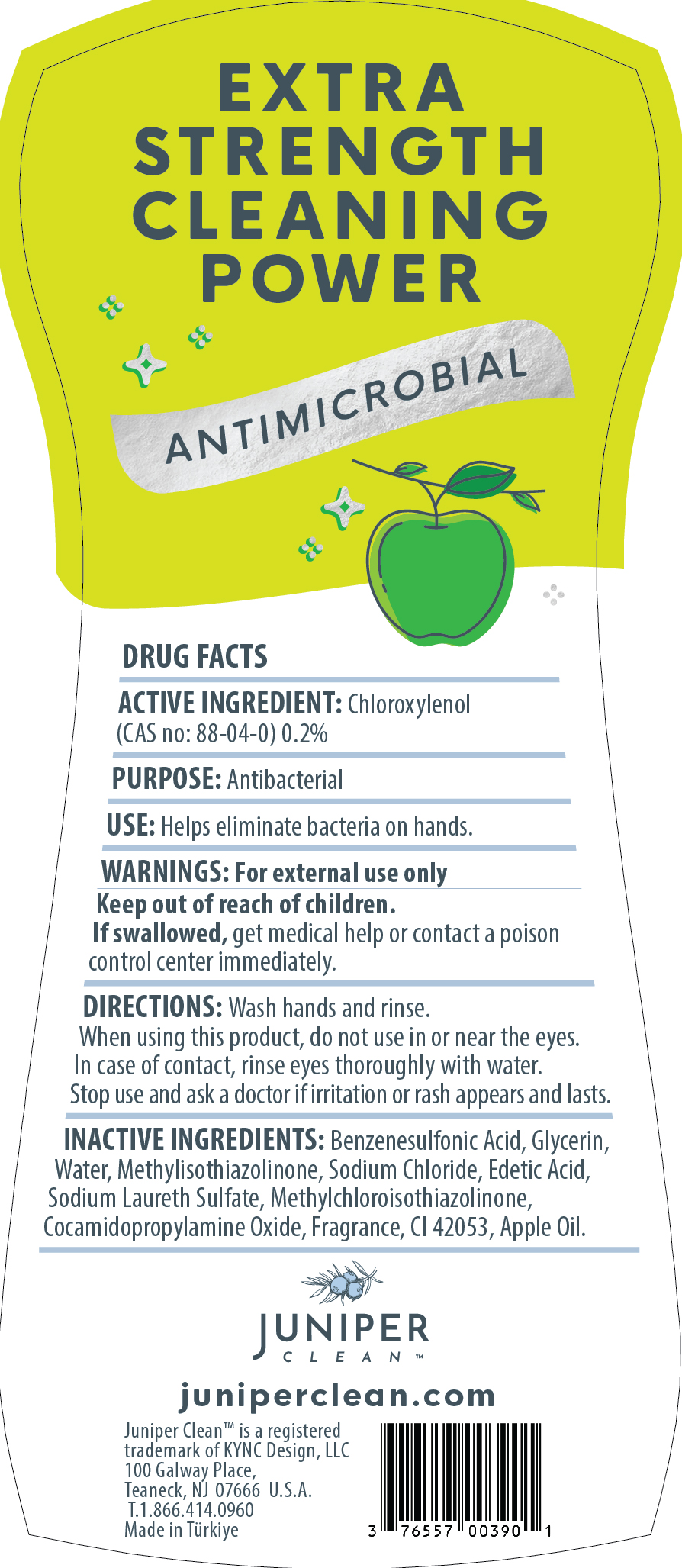
Label: JUNIPERCLEANDISHSOAP APPLE- dishwashing detergent liquid
JUNIPERCLEANDISHSOAP LEMON- dishwashing detergent liquid
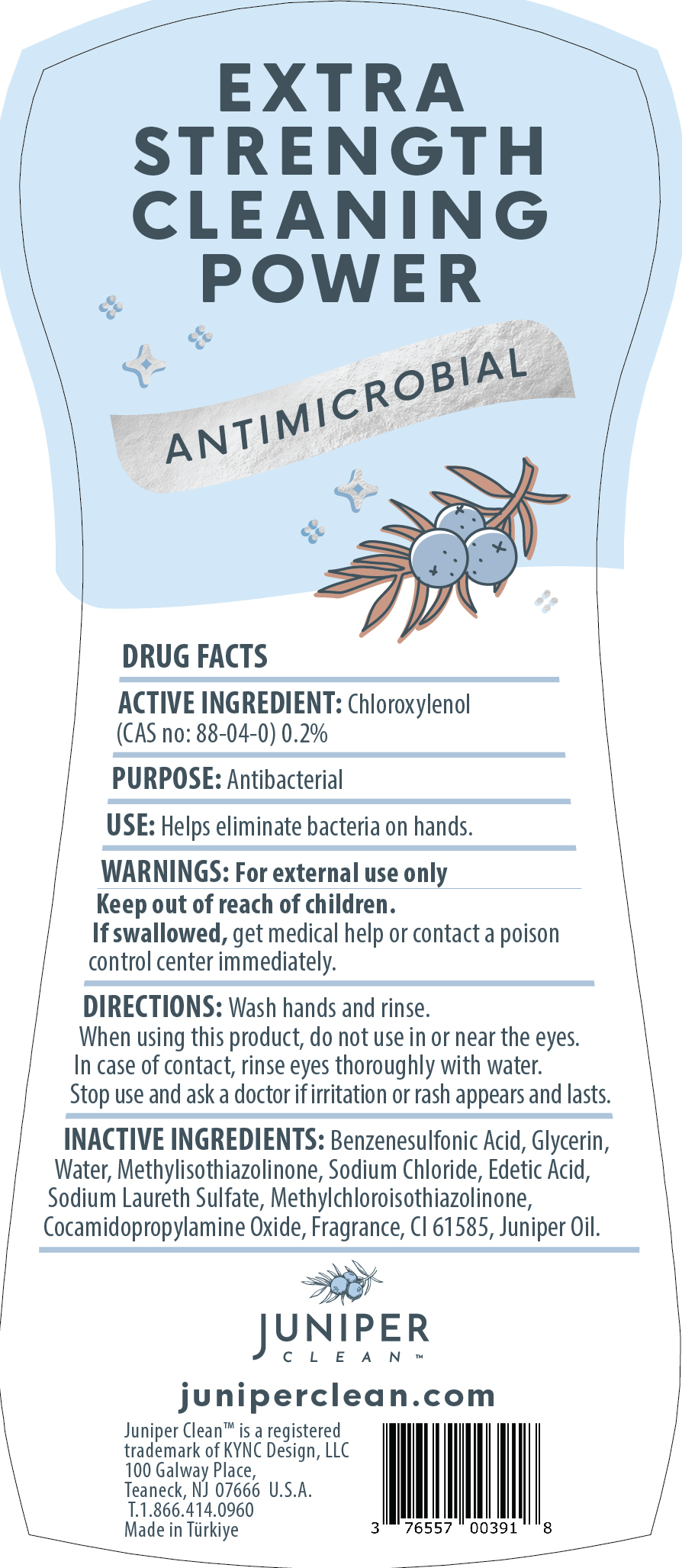
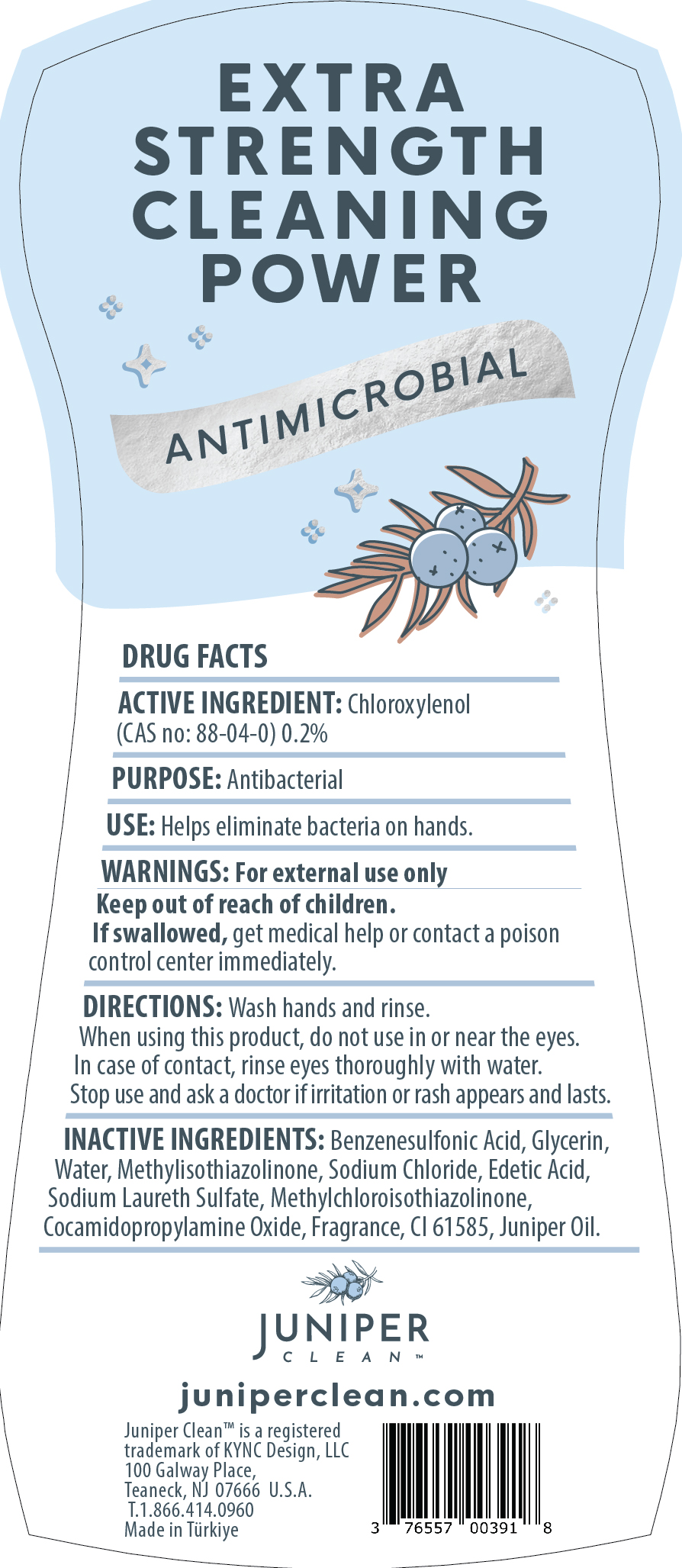
JUNIPERCLEANDISHSOAP JUNIPER- dishwashing detergent juniper forest liquid
- NDC Code(s): 76557-012-01, 76557-013-01, 76557-014-01
- Packager: KYNC DESIGN LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Use
- Warnings
- Directions
- When using this product
- Inactive Ingredients
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JUNIPERCLEANDISHSOAP APPLE
dishwashing detergent liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76557-013 Route of Administration TOPICAL, CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength P-DODECYLBENZENESULFONIC ACID (UNII: OC21S23N1O) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETIC ACID (UNII: 9G34HU7RV0) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FAST GREEN (UNII: 9J3VQ0Y6BV) APPLE (UNII: B423VGH5S9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76557-013-01 750 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/30/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/30/2023 JUNIPERCLEANDISHSOAP LEMON
dishwashing detergent liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76557-014 Route of Administration CUTANEOUS, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength P-DODECYLBENZENESULFONIC ACID (UNII: OC21S23N1O) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETIC ACID (UNII: 9G34HU7RV0) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) LEMON (UNII: 24RS0A988O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76557-014-01 750 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/30/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/30/2023 JUNIPERCLEANDISHSOAP JUNIPER
dishwashing detergent juniper forest liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76557-012 Route of Administration TOPICAL, CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength P-DODECYLBENZENESULFONIC ACID (UNII: OC21S23N1O) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETIC ACID (UNII: 9G34HU7RV0) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) JUNIPER BERRY OIL (UNII: SZH16H44UY) ACID BLUE 80 (UNII: ET8107F56D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76557-012-01 750 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/30/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/30/2023 Labeler - KYNC DESIGN LLC (039933298)