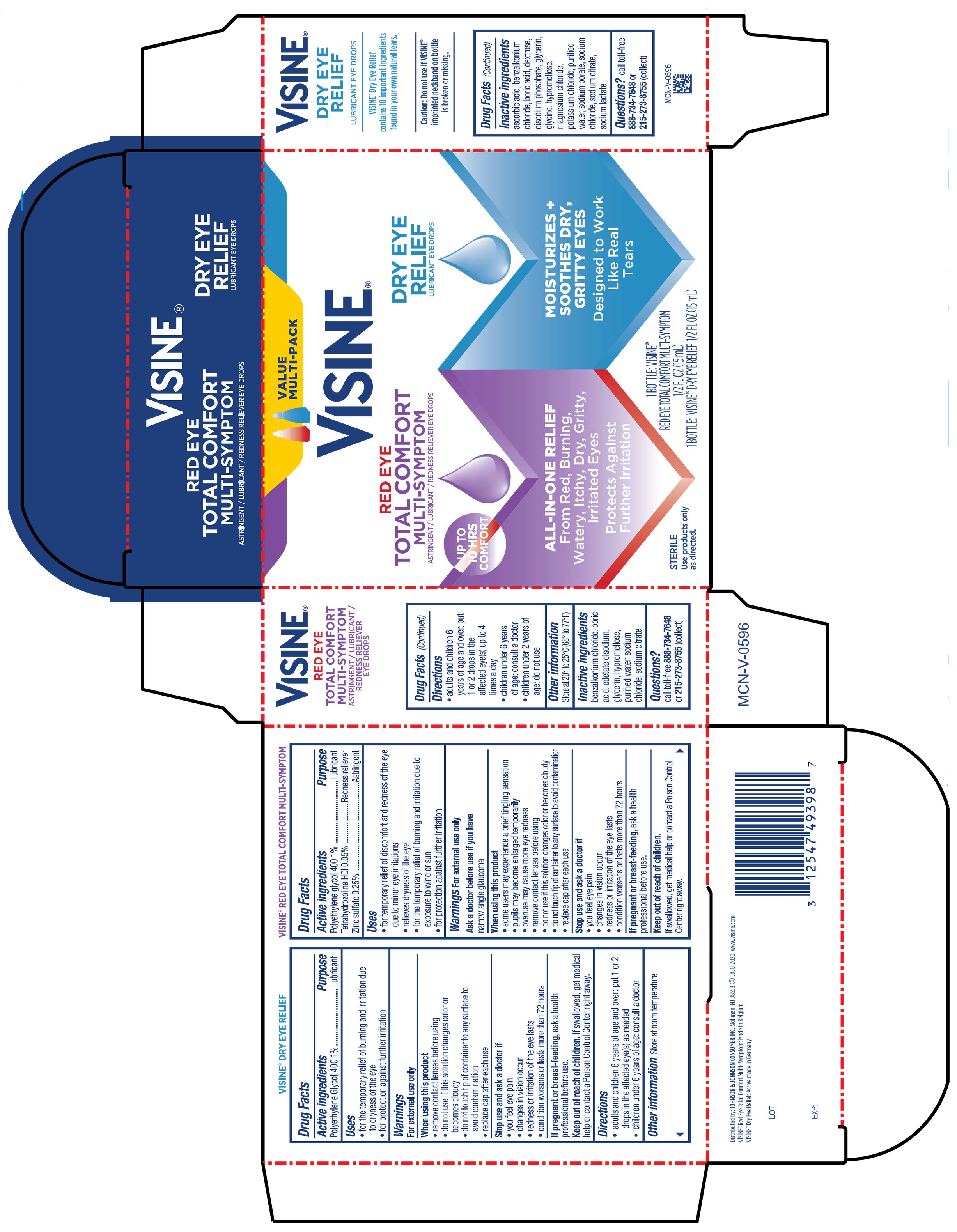
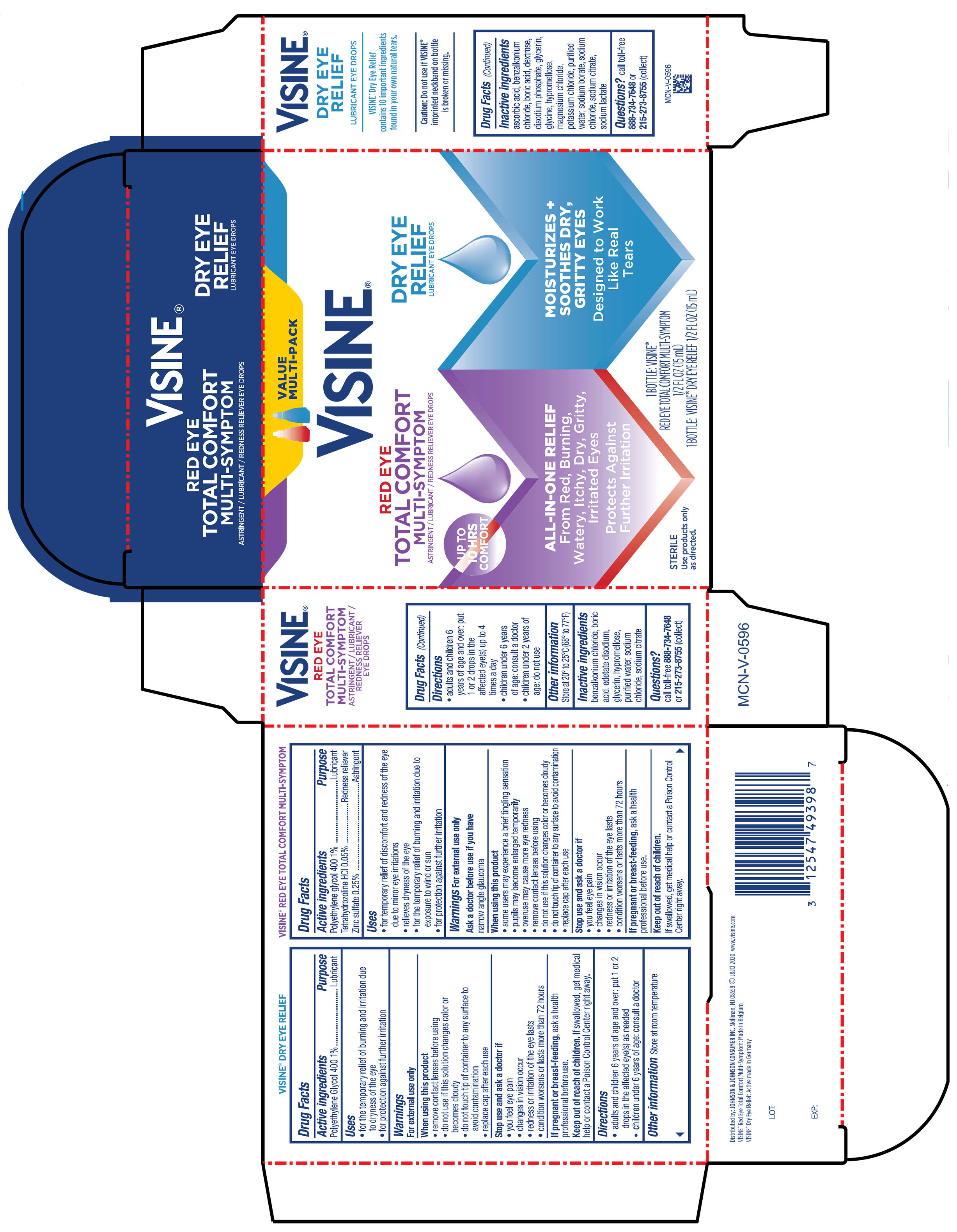
Label: VISINE RED EYE TOTAL COMFORT AND TEARS DRY EYE RELIEF- tetrahydrozoline hcl, polyethylene glycol 400, zinc sulfate kit
- NDC Code(s): 69968-0360-1, 69968-0363-1, 69968-0844-9
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
• some users may experience a brief tingling sensation
• pupils may become enlarged temporarily• overuse may cause more eye redness
• remove contact lenses before using
• do not use if this solution changes color or becomes cloudy
• do not touch tip of container to any surface to avoid contamination
• replace cap after each use
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
• remove contact lenses before using
• do not use if this solution changes color or becomes cloudy
• do not touch tip of container to any surface to avoid contamination
• replace cap after each use
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - Kit Package
VALUE
MULTI-PACK
VISINE ®
RED EYE
TOTAL COMFORT
MULTI-SYMPTOM
ASTRINGENT / LUBRICANT / REDNESS RELIEVER EYE DROPS
UP TO
10 HRS
COMFORT
ALL-IN ONE RELIEF
From Red, Burning,
Watery, Itchy, Dry, Gritty,
Irritated Eyes
Protects Against
Further Irritation
STERILE
Use products only
as directed.
DRY EYE
RELIEF
LUBRICANT EYE DROPS
MOISTURIZES +
SOOTHES DRY,
GRITTY EYES
Designed to Work
Like Real
Tears
1 BOTTLE: VISINE®
RED EYE TOTAL COMFORT MULTI-SYMPTOM
½ FL OZ (15 mL)
1 BOTTLE: VISINE® DRY EYE RELIEF ½ FL OZ (15 mL)
-
INGREDIENTS AND APPEARANCE
VISINE RED EYE TOTAL COMFORT AND TEARS DRY EYE RELIEF
tetrahydrozoline hcl, polyethylene glycol 400, zinc sulfate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0844 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0844-9 1 in 1 PACKAGE 06/08/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, DROPPER 15 mL Part 2 1 BOTTLE, DROPPER 15 mL Part 1 of 2 VISINE RED EYE TOTAL COMFORT MULTI-SYMPTOM
tetrahydrozoline hydrochloride, polyethylene glycol 400, and zinc sulfate, unspecified form solution/ dropsProduct Information Item Code (Source) NDC:69968-0360 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRAHYDROZOLINE HYDROCHLORIDE (UNII: 0YZT43HS7D) (TETRAHYDROZOLINE - UNII:S9U025Y077) TETRAHYDROZOLINE HYDROCHLORIDE 0.5 mg in 1 mL ZINC SULFATE, UNSPECIFIED FORM (UNII: 89DS0H96TB) (ZINC CATION - UNII:13S1S8SF37) ZINC SULFATE, UNSPECIFIED FORM 2.5 mg in 1 mL POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) (POLYETHYLENE GLYCOL 400 - UNII:B697894SGQ) POLYETHYLENE GLYCOL 400 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM CITRATE (UNII: 1Q73Q2JULR) EDETATE DISODIUM (UNII: 7FLD91C86K) HYPROMELLOSES (UNII: 3NXW29V3WO) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0360-1 1 in 1 CARTON 1 15 mL in 1 BOTTLE, DROPPER; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 04/27/2020 Part 2 of 2 VISINE DRY EYE RELIEF
polyethylene glycol 400 solution/ dropsProduct Information Item Code (Source) NDC:69968-0363 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) (POLYETHYLENE GLYCOL 400 - UNII:B697894SGQ) POLYETHYLENE GLYCOL 400 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) DEXTROSE (UNII: IY9XDZ35W2) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) POTASSIUM CHLORIDE (UNII: 660YQ98I10) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) BORIC ACID (UNII: R57ZHV85D4) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM LACTATE (UNII: TU7HW0W0QT) ASCORBIC ACID (UNII: PQ6CK8PD0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) HYPROMELLOSES (UNII: 3NXW29V3WO) SODIUM BORATE (UNII: 91MBZ8H3QO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0363-1 1 in 1 CARTON 1 15 mL in 1 BOTTLE, DROPPER; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 04/27/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 06/08/2020 Labeler - Johnson & Johnson Consumer Inc. (118772437)