Label: CAINETIPS- benzocaine liquid
- NDC Code(s): 70769-101-11, 70769-101-51
- Packager: J MORITA USA INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
-
WARNINGS
Do not use: if patient is allergic to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics;
exceeding recommended dosage for more than 7 days unless directed by a dentist; if sore mouth symptoms
do not improve in 7 days; if irritation, pain or redness persists or worsens; or if swelling, rash or fever develops, see a
dentist or a physician promptly. - PREGNANCY
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
HOLD WITH SOLID WHITE TIP STRAIGHT DOWN; BEND AND SNAP OPEN UPPER TIP AT COLORED RING; ALLOW MEDICINE TO FLOW DOWN.
APPLY PRODUCT TO INTENDED AREA; ALLOW IT TO REMAIN IN PLACE AT LEAST 1 MINUTE AND THEN REMOVE IT FROM MOUTH; DISPOSE AFTER APPLICATION.
USE UP TO 4 TIMES DAILY, OR AS DIRECTED BY A DENTIST. ADULT SUPERVISION REQUIRED FOR USE WITH CHILDREN UNDER 12 YEARS. CONSULT A DENTAL PROFESSIONAL FOR USE WITH CHILDREN UNDER 2 YEARS.
- INACTIVE INGREDIENTS
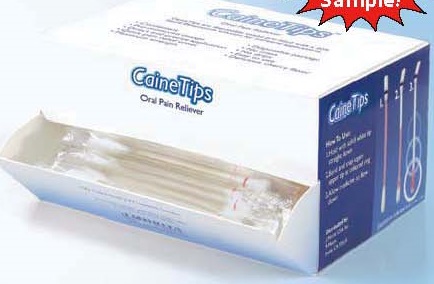
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CAINETIPS
benzocaine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70769-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 20 g in 100 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70769-101-51 100 in 1 BOX 06/06/2016 1 NDC:70769-101-11 0.15 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/06/2016 Labeler - J MORITA USA INC (021866033)