Label: NEOSPORIN ORIGINAL- bacitracin zinc, neomycin sulfate, and polymyxin b sulfate kit
- NDC Code(s): 69968-0056-1, 69968-0056-2, 69968-0843-9
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Neosporin Original Ointment
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
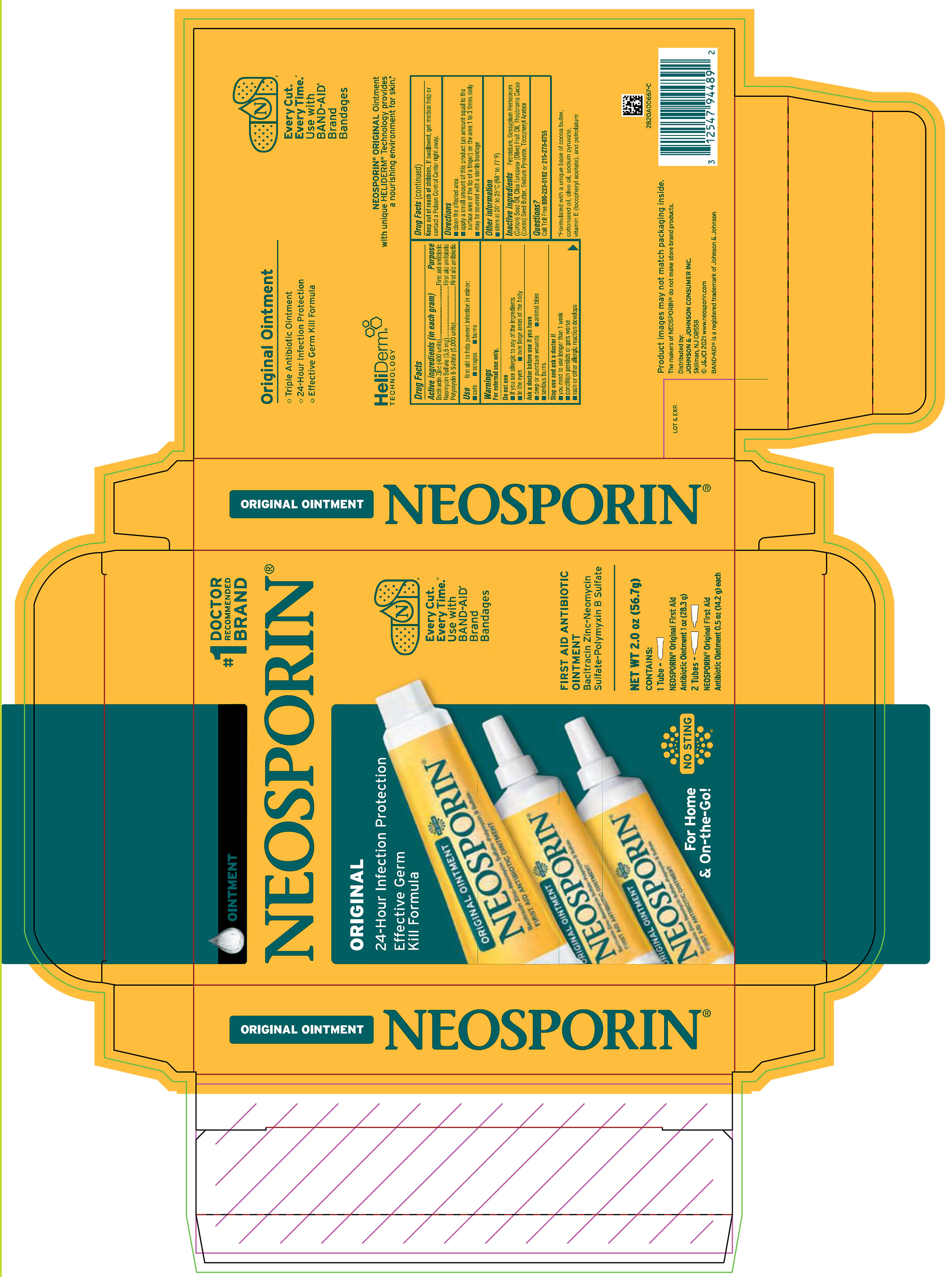
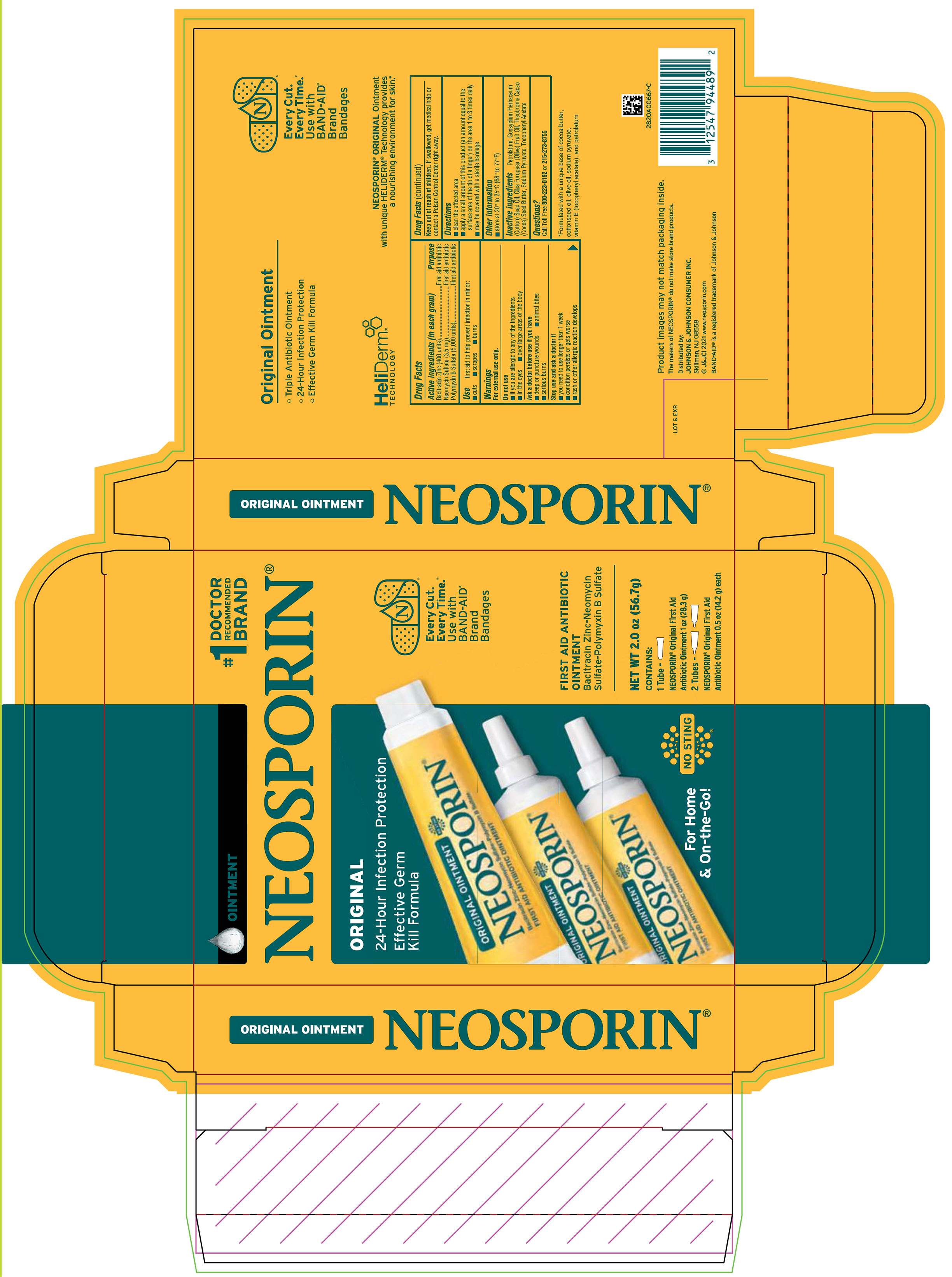
PRINCIPAL DISPLAY PANEL - Kit Package Label
OINTMENT
#1
DOCTOR
RECOMMENDED
BRANDNEOSPORIN®
ORIGINAL
24-Hour Infection Protection
Effective Germ
Kill Formula
Every Cut.
Every Time.
Use with
BAND-AID
Brand
Bandages
FIRST AID ANTIBIOTIC
OINTMENT
Bacitracin Zinc-Neomycin
Sulfate-Polymyxin B Sulfate
For Home
& On-the-Go!
NO STING
NET WT 2.0 oz (56.7 g)
CONTAINS:
1 Tube –
NEOSPORIN® Original First Aid
Antibiotic Ointment 1 oz (28.3 g)
2 Tubes –
NEOSPORIN® Original First Aid
Antibiotic Ointment 0.5 oz (14.2 g) each
-
INGREDIENTS AND APPEARANCE
NEOSPORIN ORIGINAL
bacitracin zinc, neomycin sulfate, and polymyxin b sulfate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0843 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0843-9 1 in 1 PACKAGE 06/01/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 28.3 g Part 2 1 TUBE 14.2 g Part 1 of 2 NEOSPORIN ORIGINAL
bacitracin zinc, neomycin sulfate, and polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:69968-0056 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g Inactive Ingredients Ingredient Name Strength LEVANT COTTONSEED OIL (UNII: N5CFT140R8) OLIVE OIL (UNII: 6UYK2W1W1E) COCOA BUTTER (UNII: 512OYT1CRR) SODIUM PYRUVATE (UNII: POD38AIF08) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0056-1 1 in 1 CARTON 1 28.3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 09/03/2016 Part 2 of 2 NEOSPORIN ORIGINAL
bacitracin zinc, neomycin sulfate, and polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:69968-0056 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g Inactive Ingredients Ingredient Name Strength OLIVE OIL (UNII: 6UYK2W1W1E) LEVANT COTTONSEED OIL (UNII: N5CFT140R8) COCOA BUTTER (UNII: 512OYT1CRR) SODIUM PYRUVATE (UNII: POD38AIF08) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0056-2 1 in 1 CARTON 1 14.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 09/03/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 06/01/2022 Labeler - Johnson & Johnson Consumer Inc. (118772437)