Label: DIAL FOAMING ANTIBACTERIAL DEFENSE SOOTHING WHITE TEA- benzalkonium chloride solution
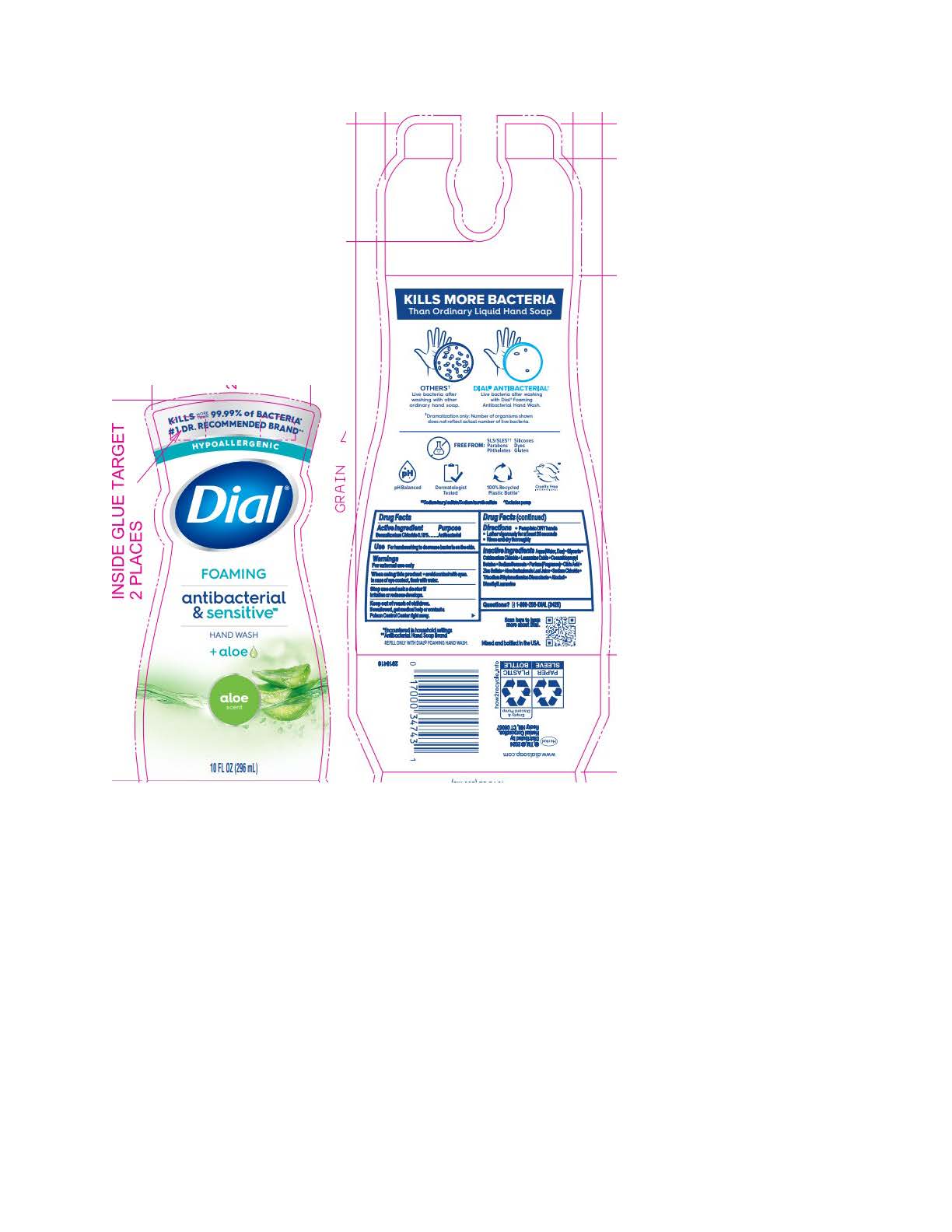
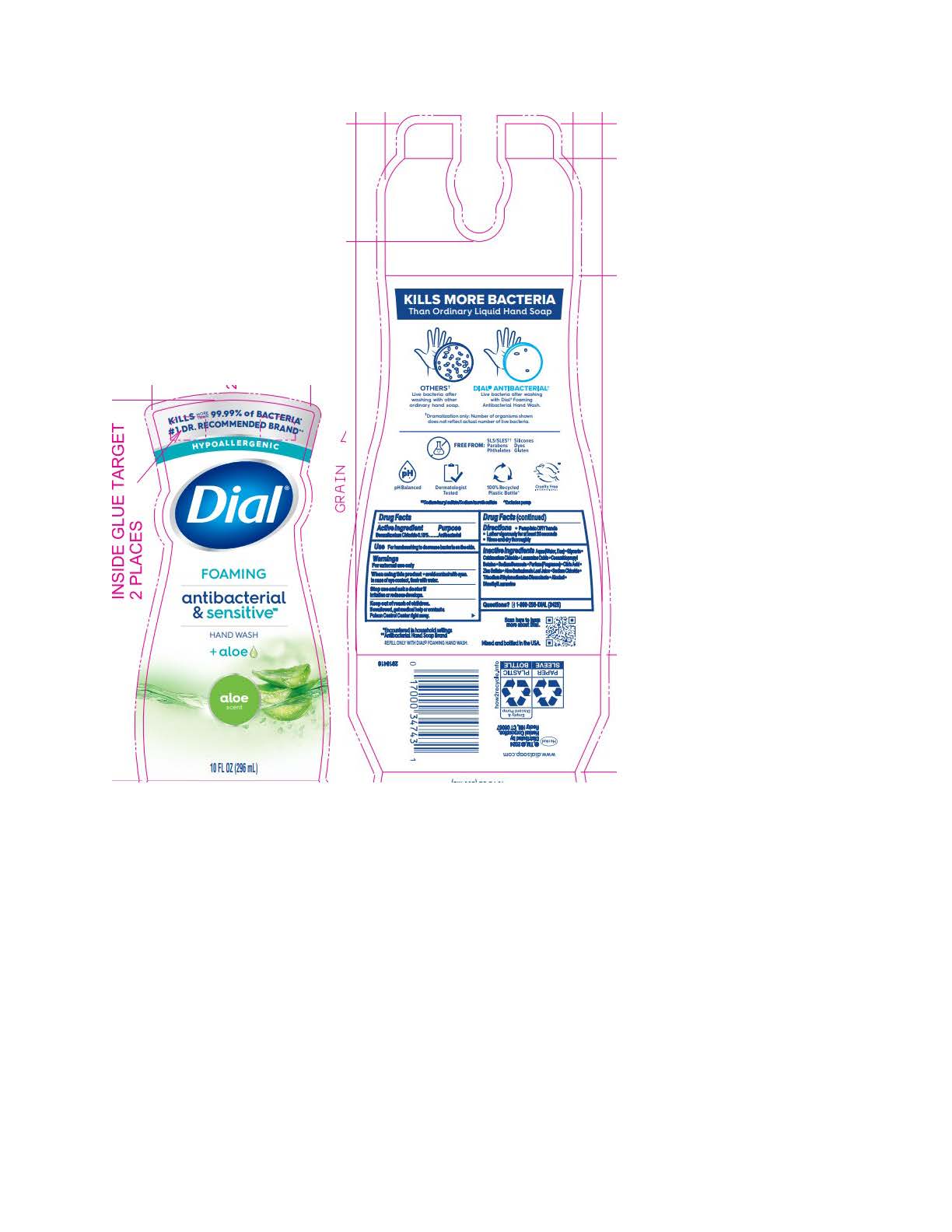
DIAL FOAMING ANTIBACTERIAL SENSITIVE ALOE- benzalkonium chloride solution
DIAL FOAMING ANTIBACTERIAL DEFENSE CITRUS SUNBURST- benzalkonium chloride solution
DIAL FOAMING ANTIBACTERIAL DEFENSE FRESH LAVENDER- benzalkonium chloride solution
DIAL FOAMING ANTIBACTERIAL DEFENSE FRESH PEAR- benzalkonium chloride solution
DIAL FOAMING ANTIBACTERIAL DEFENSE COCONUT WATER- benzalkonium chloride solution
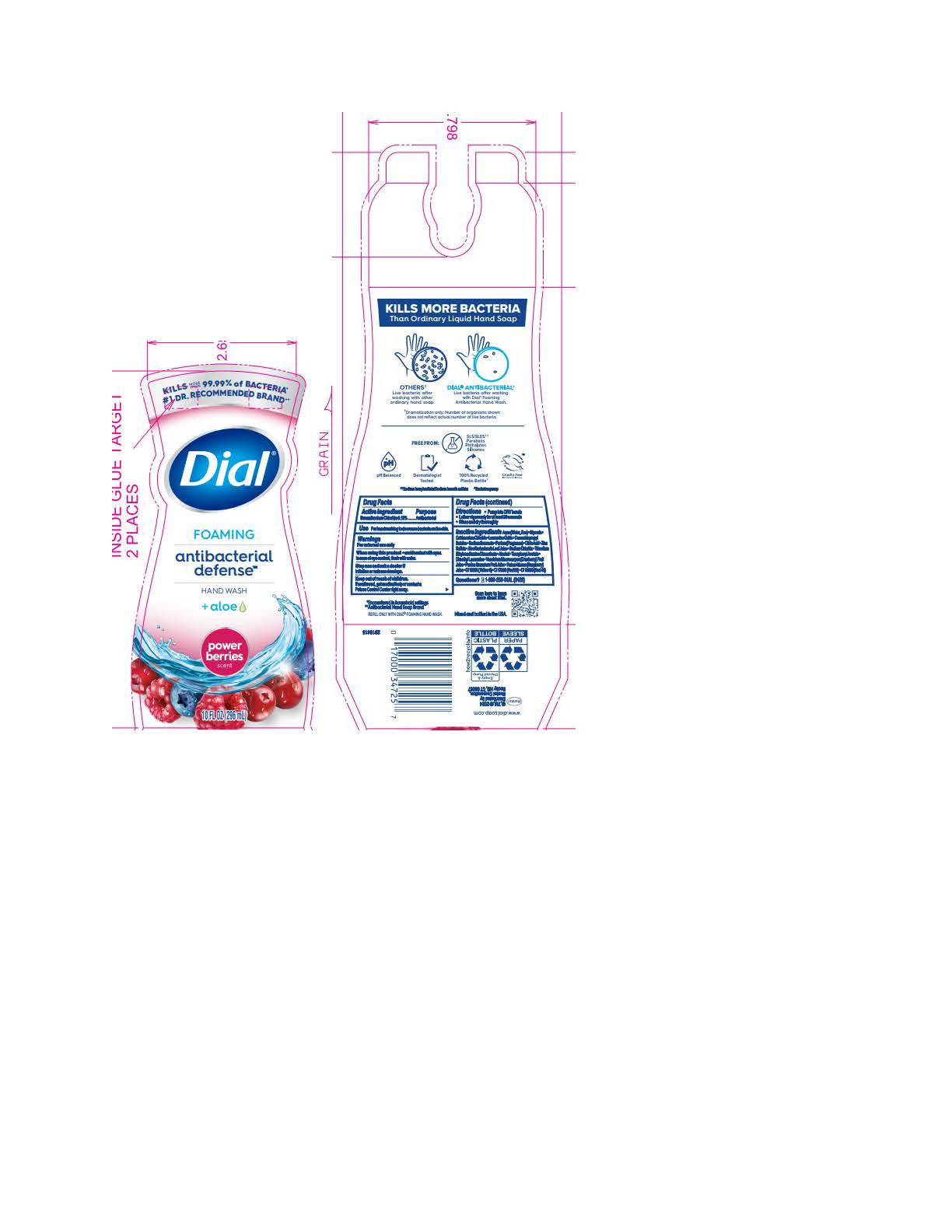
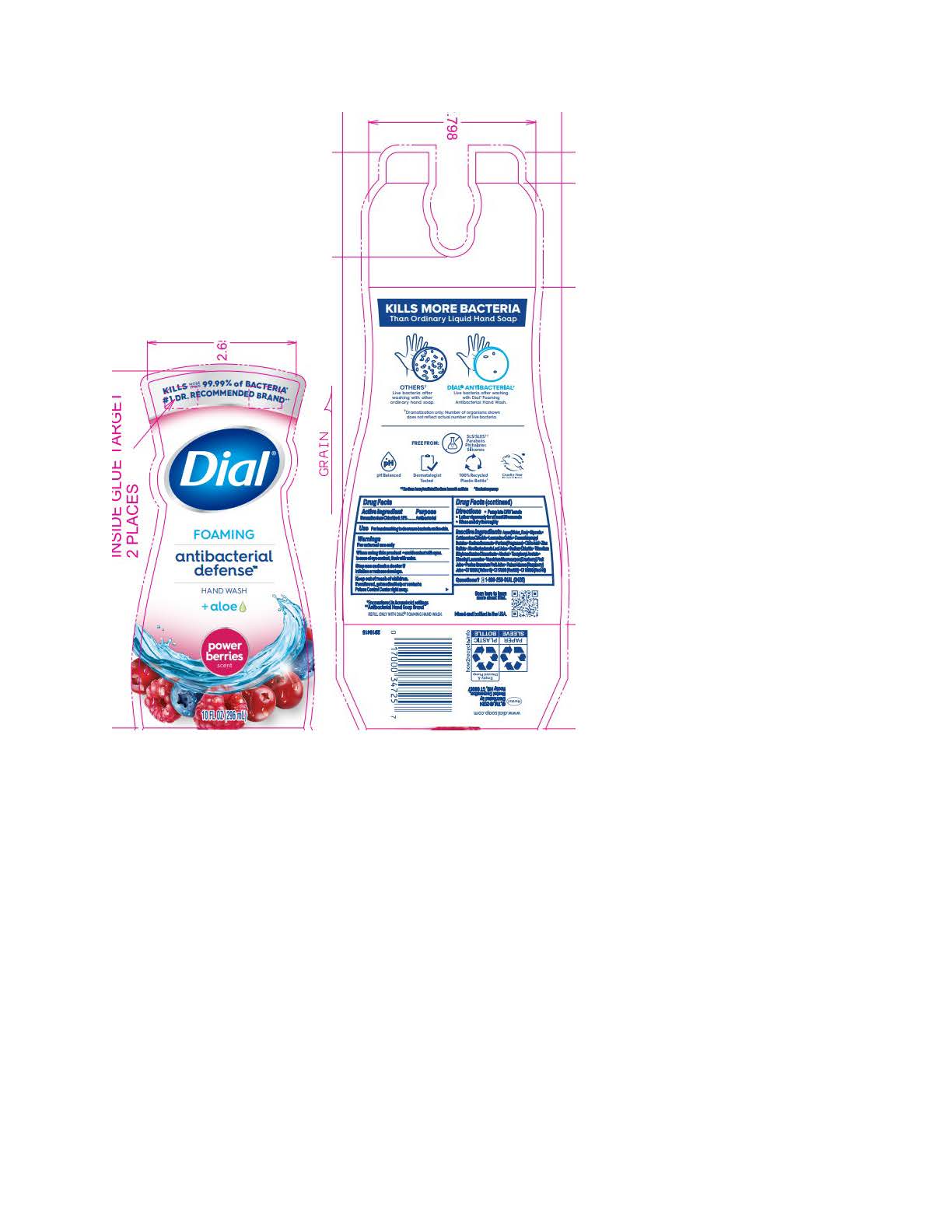
DIAL FOAMING ANTIBACTERIAL DEFENSE POWER BERRIES- benzalkonium chloride solution
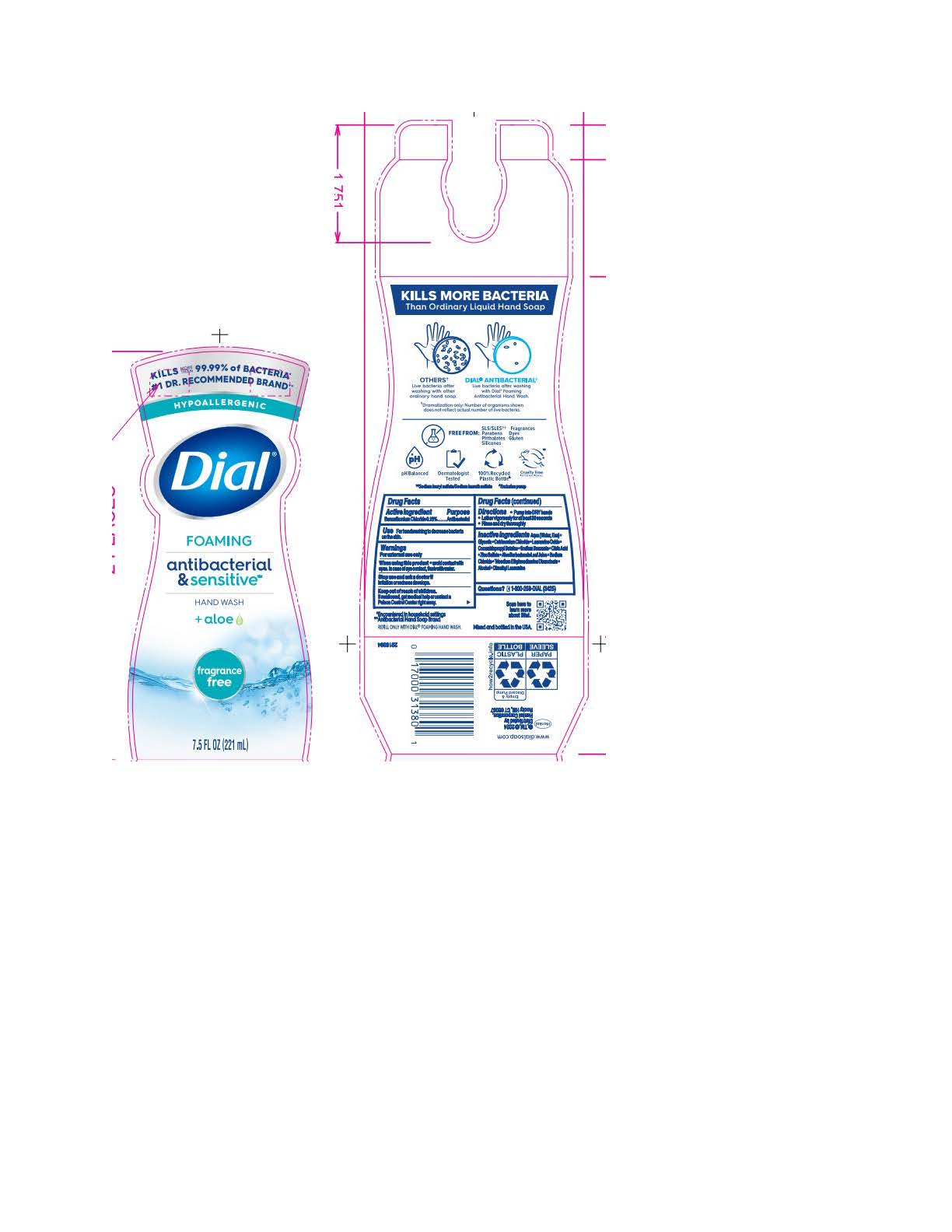
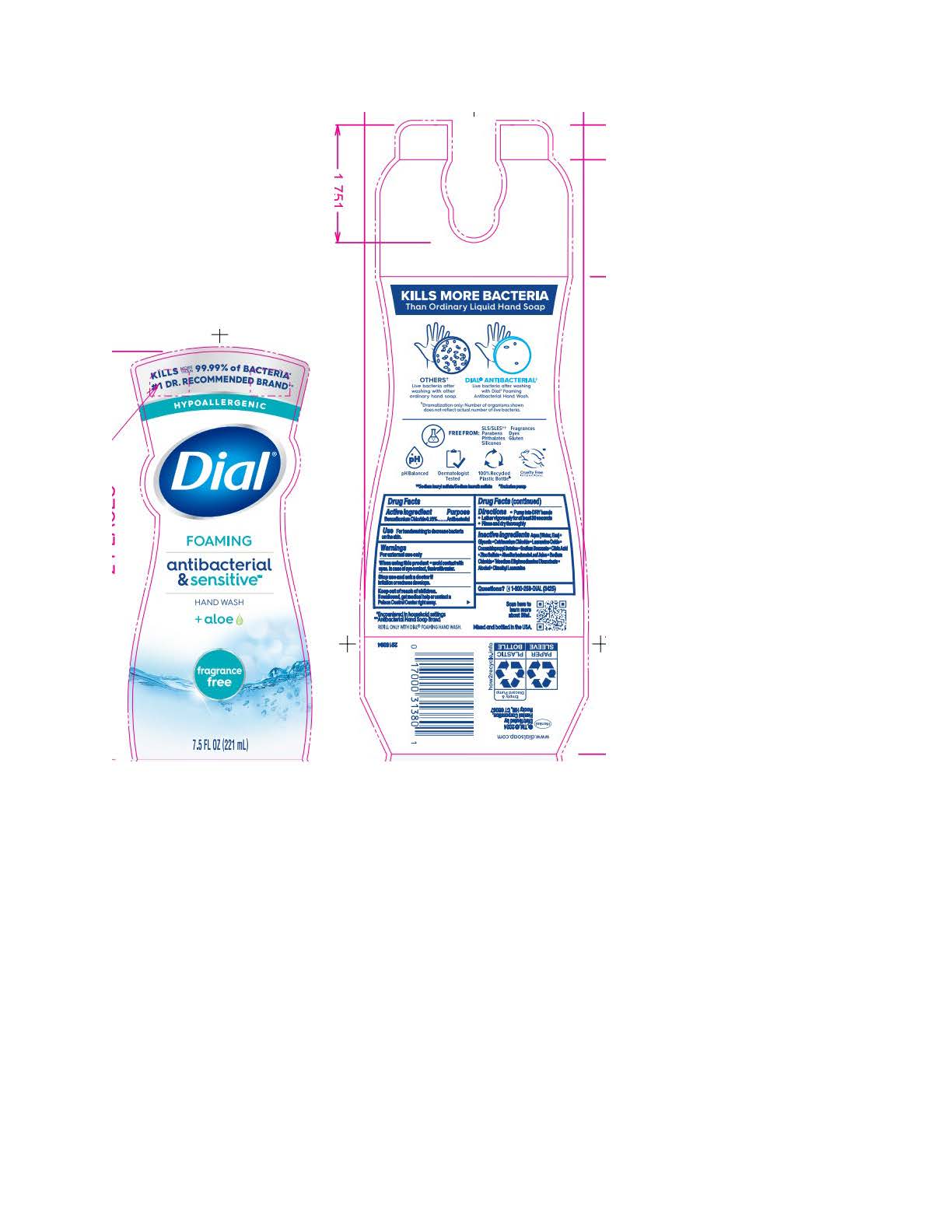
DIAL FOAMING ANTIBACTERIAL SENSITIVE FRAGRANCE FREE- benzalkonium chloride solution
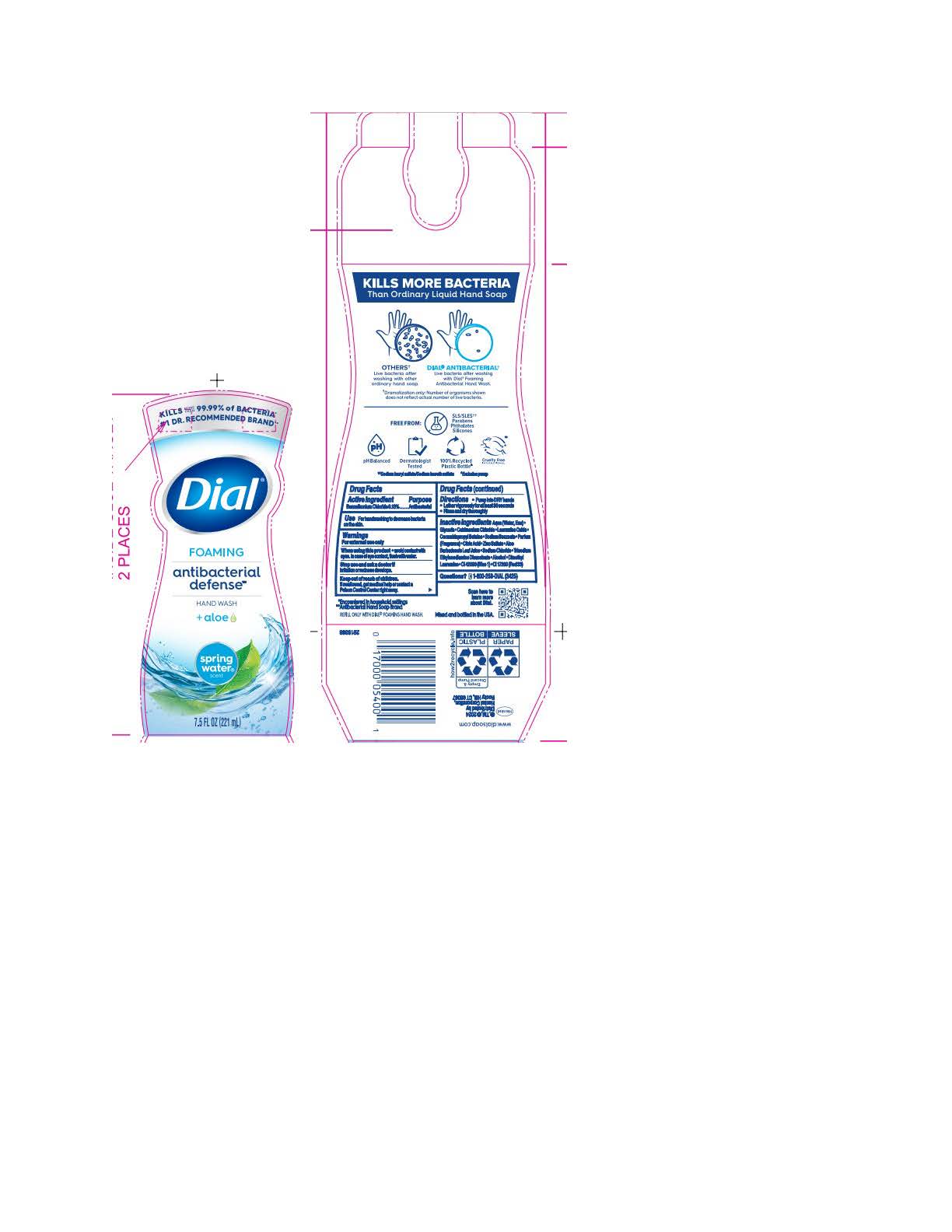
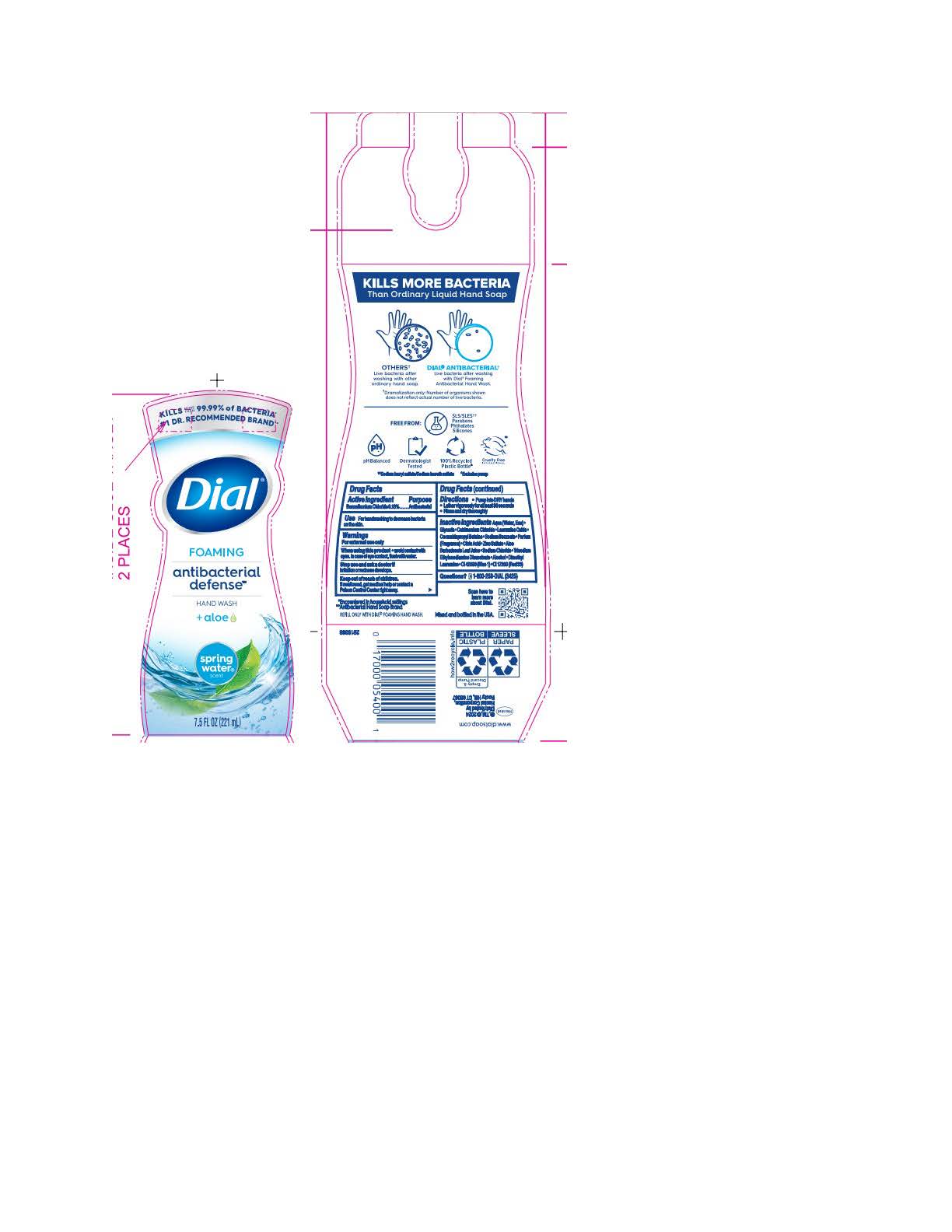
DIAL FOAMING ANTIBACTERIAL DEFENSE SPRING WATER- benzalkonium chloride solution
-
NDC Code(s):
54340-184-01,
54340-184-02,
54340-184-03,
54340-184-04, view more54340-185-01, 54340-185-02, 54340-185-03, 54340-185-04, 54340-185-05, 54340-185-06, 54340-185-07, 54340-186-01, 54340-186-02, 54340-186-03, 54340-186-04, 54340-186-05, 54340-188-01, 54340-188-02, 54340-188-03, 54340-188-05, 54340-189-01, 54340-189-02, 54340-191-01, 54340-191-02, 54340-191-03, 54340-191-04, 54340-193-01, 54340-193-02, 54340-193-03, 54340-193-04, 54340-194-01, 54340-194-02, 54340-195-01, 54340-195-02, 54340-195-03, 54340-195-04, 54340-195-05, 54340-195-06, 54340-195-07
- Packager: Henkel Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Purpose
- Uses
- Warnings
- When Using this Product
- Stop use and ask doctor if
- Keep out of reach of children.
- Directions
-
Inactive Ingredients
Fresh Lavender
Aqua (Water, Eau) · Glycerin · Cetrimonium Chloride · Lauramine Oxide · Cocamidopropyl Betaine · Sodium Benzoate ·
Parfum (Fragrance) · Citric Acid · Zinc Sulfate · Aloe Barbadensis Leaf Juice · Sodium Chloride · Trisodium Ethylenediamine Disuccinate · Alcohol · Dimethyl Lauramine · CI 60730 (Ext. Violet 2)Coconut Water
Inactive ingredients: Aqua (Water, Eau) · Glycerin · Cetrimonium Chloride · Lauramine Oxide · Cocamidopropyl Betaine · Sodium Benzoate · Parfum (Fragrance) · Citric Acid · Zinc Sulfate · Aloe Barbadensis Leaf Juice · Sodium Chloride · Trisodium Ethylenediamine Disuccinate · Alcohol · Dimethyl Lauramine · CI 42090 (Blue 1) · CI 17200 (Red 33)
Fresh Pear
Inactive ingredients: Aqua (Water, Eau) · Glycerin · Cetrimonium Chloride · Lauramine Oxide · Cocamidopropyl Betaine · Sodium Benzoate · Parfum (Fragrance) · Citric Acid · Zinc Sulfate · Aloe Barbadensis Leaf Juice · Sodium Chloride · Trisodium Ethylenediamine Disuccinate · Alcohol · Dimethyl Lauramine · CI 47005 (Yellow 10) · CI 42053 (Green 3)
Soothing White Tea
Aqua (Water, Eau) · Glycerin · Cetrimonium Chloride · Lauramine Oxide · Cocamidopropyl Betaine · Sodium Benzoate ·
Parfum (Fragrance) · Citric Acid · Zinc Sulfate · Aloe Barbadensis Leaf Juice · Sodium Chloride · Trisodium Ethylenediamine Disuccinate · Alcohol · Dimethyl Lauramine · CI 42090 (Blue 1) · CI 17200 (Red 33)Sensitive Aloe
Inactive ingredients: Aqua (Water, Eau) · Glycerin · Cetrimonium Chloride · Lauramine Oxide · Cocamidopropyl Betaine · Sodium Benzoate · Parfum (Fragrance) · Citric Acid · Zinc Sulfate · Aloe Barbadensis Leaf Juice · Sodium Chloride · Trisodium Ethylenediamine Disuccinate · Alcohol · Dimethyl Lauramine
Citrus Sunburst
Aqua (Water, Eau) · Glycerin · Cetrimonium Chloride · Lauramine Oxide · Cocamidopropyl Betaine · Sodium Benzoate ·
Parfum (Fragrance) · Citric Acid · Zinc Sulfate · Aloe Barbadensis Leaf Juice · Sodium Chloride · Trisodium Ethylenediamine Disuccinate · Alcohol · Dimethyl Lauramine · CI 15985 (Yellow 6) · CI 14700 (Red 4)Power Berries
Aqua (Water, Eau) · Glycerin · Cetrimonium Chloride · Lauramine Oxide · Cocamidopropyl Betaine · Sodium Benzoate ·
Parfum (Fragrance) · Citric Acid · Zinc Sulfate · Aloe Barbadensis Leaf Juice · Sodium Chloride · Trisodium Ethylenediamine Disuccinate · Alcohol · Tocopheryl Acetate · Dimethyl Lauramine · Vaccinium Macrocarpon (Cranberry) Fruit Juice · Punica Granatum Fruit Juice · Rubus Idaeus (Raspberry) Juice · CI 15985 (Yellow 6) · CI 17200 (Red 33) · CI 16035 (Red 40)Spring Water
Aqua (Water, Eau) · Glycerin · Cetrimonium Chloride · Lauramine Oxide · Cocamidopropyl Betaine · Sodium Benzoate · Parfum (Fragrance) · Citric Acid · Zinc Sulfate · Aloe Barbadensis Leaf Juice · Sodium Chloride · Trisodium Ethylenediamine Disuccinate · Alcohol · Dimethyl Lauramine · CI 42090 (Blue 1) · CI 17200 (Red 33)
Sensitive & Fragrance Free
Aqua (Water, Eau) · Glycerin · Cetrimonium Chloride · Lauramine Oxide · Cocamidopropyl Betaine · Sodium Benzoate ·
Citric Acid · Zinc Sulfate · Aloe Barbadensis Leaf Juice · Sodium Chloride · Trisodium Ethylenediamine Disuccinate · Alcohol · Dimethyl Lauramine - Questions
- Legal Entity
- ^Encountered in household settings
- Indications & Usage
- Topical Liquid
- Front and the back of the pack
-
INGREDIENTS AND APPEARANCE
DIAL FOAMING ANTIBACTERIAL DEFENSE SOOTHING WHITE TEA
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-186 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.0099 g in 100 mL WATER (UNII: 059QF0KO0R) 95.627 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1.512 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 0.999 g in 100 mL LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 0.597 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 0.391 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.2 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.11 g in 100 mL ZINC SULFATE (UNII: 89DS0H96TB) 0.1 g in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.0997 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.072 g in 100 mL TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) 0.0185 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.000009 g in 100 mL D&C RED NO. 33 (UNII: 9DBA0SBB0L) 0.000008 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-186-01 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 2 NDC:54340-186-02 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 3 NDC:54340-186-03 887 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2023 4 NDC:54340-186-04 6 in 1 CARTON 05/01/2023 4 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 5 NDC:54340-186-05 8 in 1 CARTON 05/01/2023 5 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2023 DIAL FOAMING ANTIBACTERIAL SENSITIVE ALOE
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-193 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.0099 g in 100 mL WATER (UNII: 059QF0KO0R) 95.59 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1.512 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 0.999 g in 100 mL LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 0.597 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 0.391 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.2 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.11 g in 100 mL ZINC SULFATE (UNII: 89DS0H96TB) 0.1 g in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.0997 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.072 g in 100 mL TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) 0.0185 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-193-01 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 2 NDC:54340-193-02 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 3 NDC:54340-193-03 6 in 1 CARTON 05/01/2023 3 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 4 NDC:54340-193-04 8 in 1 CARTON 05/01/2023 4 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2023 DIAL FOAMING ANTIBACTERIAL DEFENSE CITRUS SUNBURST
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-188 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 95.553 g in 100 mL DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.0099 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1.51 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 0.999 g in 100 mL LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 0.597 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 0.391 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.2 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.11 g in 100 mL ZINC SULFATE (UNII: 89DS0H96TB) 0.1 g in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.0997 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.072 g in 100 mL TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) 0.0185 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL FD&C YELLOW NO. 6 (UNII: H77VEI93A8) 0.00022 g in 100 mL FD&C RED NO. 4 (UNII: X3W0AM1JLX) 0.00014 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-188-01 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 2 NDC:54340-188-02 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 3 NDC:54340-188-03 6 in 1 CARTON 05/01/2023 3 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 4 NDC:54340-188-05 8 in 1 CARTON 05/01/2023 4 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2023 DIAL FOAMING ANTIBACTERIAL DEFENSE FRESH LAVENDER
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-189 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 95.5 g in 100 mL DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.0099 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1.51 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 0.999 g in 100 mL LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 0.597 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 0.391 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.2 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.11 g in 100 mL ZINC SULFATE (UNII: 89DS0H96TB) 0.1 g in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.0997 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.072 g in 100 mL TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) 0.0185 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL D&C VIOLET NO. 2 (UNII: 350KA7O6HK) 0.000175 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-189-01 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 2 NDC:54340-189-02 6 in 1 CARTON 05/01/2023 2 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2023 DIAL FOAMING ANTIBACTERIAL DEFENSE FRESH PEAR
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-185 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 0.597 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 0.391 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.2 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.11 g in 100 mL ZINC SULFATE (UNII: 89DS0H96TB) 0.1 g in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.0997 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.072 g in 100 mL TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) 0.0185 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) 0.000035 g in 100 mL FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) 0.000027 g in 100 mL DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.0099 g in 100 mL WATER (UNII: 059QF0KO0R) 95.587 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1.515 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 0.999 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-185-01 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 2 NDC:54340-185-02 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 3 NDC:54340-185-03 887 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2023 4 NDC:54340-185-04 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2023 5 NDC:54340-185-05 6 in 1 CARTON 05/01/2023 5 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 6 NDC:54340-185-06 3 in 1 CARTON 05/01/2023 6 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 7 NDC:54340-185-07 8 in 1 CARTON 05/01/2023 7 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2023 DIAL FOAMING ANTIBACTERIAL DEFENSE COCONUT WATER
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-184 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.0099 g in 100 mL WATER (UNII: 059QF0KO0R) 95.62 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1.512 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 0.999 g in 100 mL LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 0.597 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 0.391 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.2 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.11 g in 100 mL ZINC SULFATE (UNII: 89DS0H96TB) 0.1 g in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.0997 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.072 g in 100 mL TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) 0.0185 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.000023 g in 100 mL D&C RED NO. 33 (UNII: 9DBA0SBB0L) 0.00001 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-184-01 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 2 NDC:54340-184-02 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 3 NDC:54340-184-03 8 in 1 CARTON 05/01/2023 3 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 4 NDC:54340-184-04 6 in 1 CARTON 05/01/2023 4 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2023 DIAL FOAMING ANTIBACTERIAL DEFENSE POWER BERRIES
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-191 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength RUBUS IDAEUS WHOLE (UNII: 7GOM628K2Z) 0.0015 g in 100 mL VACCINIUM MACROCARPON WHOLE (UNII: D11KO7O2DX) 0.0015 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 0.999 g in 100 mL DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.0099 g in 100 mL WATER (UNII: 059QF0KO0R) 95.5 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1.53 g in 100 mL LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 0.597 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 0.391 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.2 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.11 g in 100 mL ZINC SULFATE (UNII: 89DS0H96TB) 0.1 g in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.0997 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.072 g in 100 mL TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) 0.0185 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL FD&C RED NO. 40 (UNII: WZB9127XOA) 0.000014 g in 100 mL D&C RED NO. 33 (UNII: 9DBA0SBB0L) 0.000008 g in 100 mL FD&C YELLOW NO. 6 (UNII: H77VEI93A8) 0.000064 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-191-01 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 2 NDC:54340-191-02 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 3 NDC:54340-191-04 8 in 1 CARTON 05/01/2023 3 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 4 NDC:54340-191-03 6 in 1 CARTON 05/01/2023 4 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2023 DIAL FOAMING ANTIBACTERIAL SENSITIVE FRAGRANCE FREE
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-194 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.0099 g in 100 mL WATER (UNII: 059QF0KO0R) 95.7 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1.515 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 0.999 g in 100 mL LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 0.597 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 0.391 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.2 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.11 g in 100 mL ZINC SULFATE (UNII: 89DS0H96TB) 0.1 g in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.0997 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.072 g in 100 mL TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) 0.0185 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-194-01 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 2 NDC:54340-194-02 6 in 1 CARTON 05/01/2023 2 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2023 DIAL FOAMING ANTIBACTERIAL DEFENSE SPRING WATER
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-195 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.0099 g in 100 mL WATER (UNII: 059QF0KO0R) 95.6 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1.512 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 0.999 g in 100 mL LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 0.597 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 0.391 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.2 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.11 g in 100 mL ZINC SULFATE (UNII: 89DS0H96TB) 0.1 g in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.0997 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.072 g in 100 mL TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) 0.0185 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.000054 g in 100 mL D&C RED NO. 33 (UNII: 9DBA0SBB0L) 0.00003 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-195-01 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 2 NDC:54340-195-02 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2023 3 NDC:54340-195-03 887 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2023 4 NDC:54340-195-04 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2023 5 NDC:54340-195-05 6 in 1 CARTON 05/01/2023 5 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 6 NDC:54340-195-06 8 in 1 CARTON 05/01/2023 6 296 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 7 NDC:54340-195-07 3 in 1 CARTON 05/01/2023 7 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2023 Labeler - Henkel Corporation (080887708)