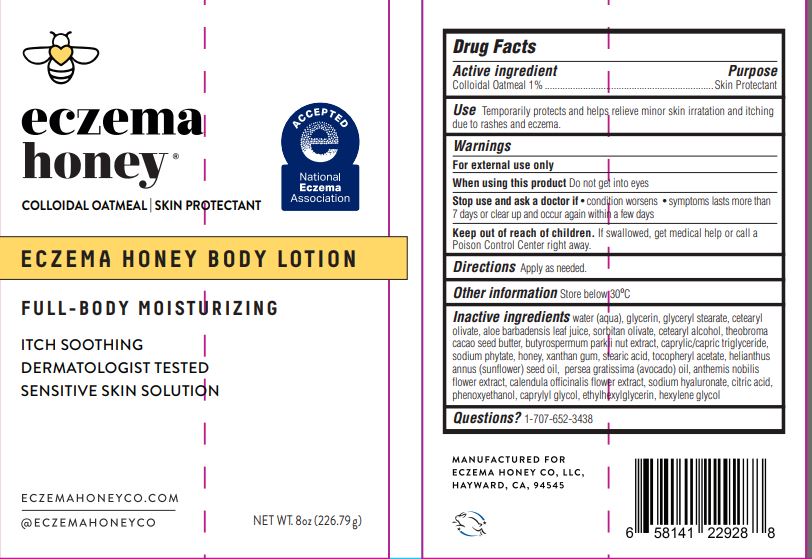
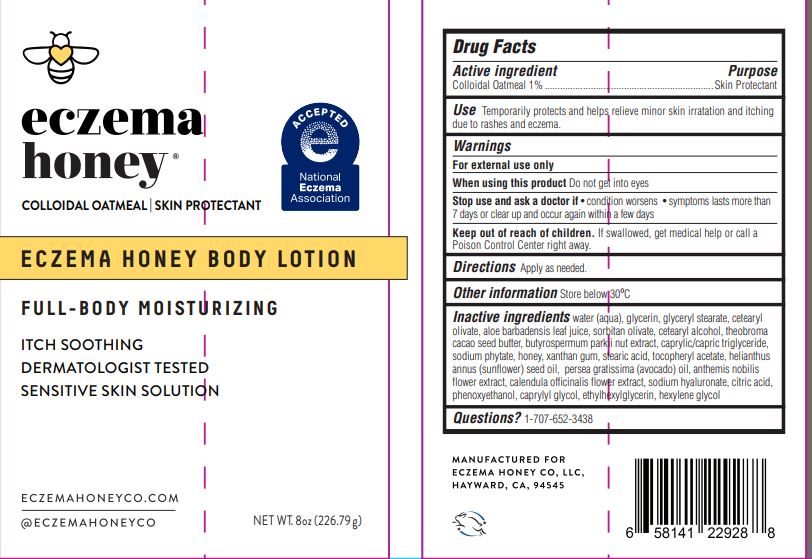
Label: ECZEMAHONEYBODYLOTION- colloidal oatmeal lotion
- NDC Code(s): 62742-4234-1, 62742-4234-2
- Packager: Allure Labs
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients
water (aqua), glycerin, glyceryl stearate, cetearyl olivate, aloe barbadensis leaf juice, soritan olivate, cetearyl alcohol, theobroma cacao seed butter, butyrospermum parkii nut extract, caprylic/capric triglyceride, sodium phytate, honey, xanthan gum, stearic acid, tocopheryl acetate, helianthus annus (sunflower) seed oil, persea gratissima (avocado) oil, anthemis nobilis flower extract, calendula officinalis flower extract, sodium hyaluronate, citric acid, phenoxyethanol, caprylyl glycol, ethylhexylglycerin, hexylene glycol
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ECZEMAHONEYBODYLOTION
colloidal oatmeal lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62742-4234 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 1 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) CETEARYL OLIVATE (UNII: 58B69Q84JO) SHEANUT OIL (UNII: O88E196QRF) STEARIC ACID (UNII: 4ELV7Z65AP) SUNFLOWER OIL (UNII: 3W1JG795YI) AVOCADO OIL (UNII: 6VNO72PFC1) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYLENE GLYCOL (UNII: KEH0A3F75J) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) WATER (UNII: 059QF0KO0R) SORBITAN OLIVATE (UNII: MDL271E3GR) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) HONEY (UNII: Y9H1V576FH) XANTHAN GUM (UNII: TTV12P4NEE) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ALOE VERA LEAF (UNII: ZY81Z83H0X) COCOA BUTTER (UNII: 512OYT1CRR) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHYTATE SODIUM (UNII: 88496G1ERL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62742-4234-1 226.79 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 11/18/2023 2 NDC:62742-4234-2 453.59 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 11/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/18/2023 Labeler - Allure Labs (926831603) Registrant - Allure Labs (926831603) Establishment Name Address ID/FEI Business Operations Allure Labs 926831603 manufacture(62742-4234)