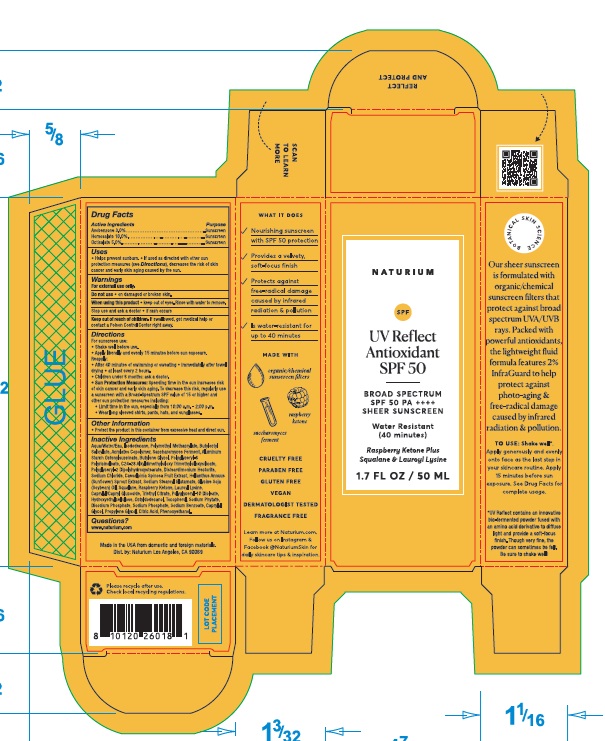
Label: NATURIUM UV REFLECT ANTIOXIDANT SPF 50- homosalate, octisalate, avobenzone lotion
- NDC Code(s): 76354-125-01
- Packager: e.l.f. Cosmetics, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
For sunscreen use:
- Shake well before use.
- Apply liberally and evenly 15 minutes before sun exposure.
Reapply:
- After 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours.
- Children under 6 months: ask a doctor.
- Sun Protection Measures: Spending time in the sun increases risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with a Broad-Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10:00 a.m. - 2:00 p.m.
- Wear long sleeved shirts, pants, hats, and sunglasses.
- Other Information
-
Inactive Ingredients
Aqua/Water/Eau, Isododecane, Polymethyl Methacryate, Butyloctyl Salicylate, Acrylates Copolymer, Saccharomyces Ferment, Aluminum Starch Octenylsuccinate, Butylene Glycol, Polyglyceryl-6 Polyricinoleate, C24-28 Alkyldimethylsiloxy Trimethylsiloxysilicate, Polyglyceryl-2 Dipolyhydroxystearate, Disteardimonium Hectorite, Sodium Chloride, Caesalpinia Spinosa Fruit Extract, Helianthus Annuus (Sunflower) Sprout Extract, Sodium Stearoyl Glutamate, Glycine Soja (Soybean) Oil, Squalane, Raspberry Ketone, Lauroyl Lysine, Caprylyl/Capryl Glucoside, Triethyl Citrate, Polyglyceryl-10 Dioleate, Hydroxyethylcellulose, Octyldodecanol, Tocopherol, Sodium Phytate, Disodium Phosphate, Sodium Phosphate, Sodium Benzoate, Caprylyl Glycol, Propylene Glycol, Citric Acid, Phenoxyethanol.
- Questions?
- Product Packaging
-
INGREDIENTS AND APPEARANCE
NATURIUM UV REFLECT ANTIOXIDANT SPF 50
homosalate, octisalate, avobenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76354-125 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength PHYTATE SODIUM (UNII: 88496G1ERL) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE (UNII: SE337SVY37) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) SODIUM BENZOATE (UNII: OJ245FE5EU) 4-(P-HYDROXYPHENYL)-2-BUTANONE (UNII: 7QY1MH15BG) SQUALANE (UNII: GW89575KF9) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ISODODECANE (UNII: A8289P68Y2) SOYBEAN OIL (UNII: 241ATL177A) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM CHLORIDE (UNII: 451W47IQ8X) POLYGLYCERYL-10 DIOLEATE (UNII: 598RES7AXX) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) PHENOXYETHANOL (UNII: HIE492ZZ3T) LAUROYL LYSINE (UNII: 113171Q70B) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) OCTYLDODECANOL (UNII: 461N1O614Y) HELIANTHUS ANNUUS SPROUT (UNII: 4P26HG1S5W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76354-125-01 1 in 1 CARTON 04/10/2023 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/10/2023 Labeler - e.l.f. Cosmetics, Inc (093902816)