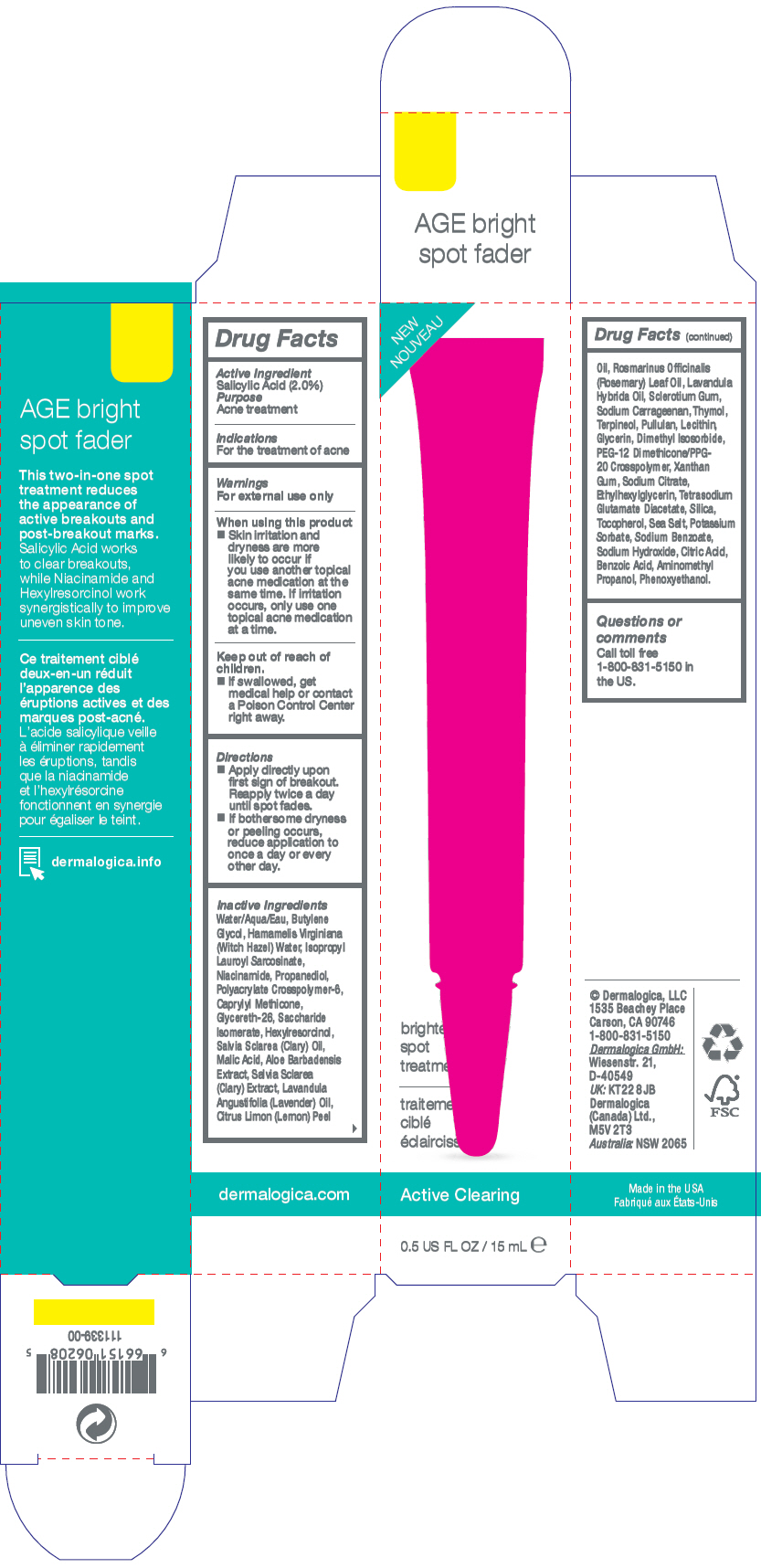
Label: AGE BRIGHT SPOT FADER- salicylic acid gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 68479-806-00, 68479-806-01, 68479-806-02 - Packager: Dermalogica, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 18, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Indications
- Warnings
- Directions
-
Inactive Ingredients
Water/Aqua/Eau, Butylene Glycol, Hamamelis Virginiana (Witch Hazel) Water, Isopropyl Lauroyl Sarcosinate, Niacinamide, Propanediol, Polyacrylate Crosspolymer-6, Caprylyl Methicone, Glycereth-26, Saccharide Isomerate, Hexylresorcinol, Salvia Sclarea (Clary) Oil, Malic Acid, Aloe Barbadensis Extract, Salvia Sclarea (Clary) Extract, Lavandula Angustifolia (Lavender) Oil, Citrus Limon (Lemon) Peel Oil, Rosmarinus Officinalis (Rosemary) Leaf Oil, Lavandula Hybrida Oil, Sclerotium Gum, Sodium Carrageenan, Thymol, Terpineol, Pullulan, Lecithin, Glycerin, Dimethyl Isosorbide, PEG-12 Dimethicone/PPG-20 Crosspolymer, Xanthan Gum, Sodium Citrate, Ethylhexylglycerin, Tetrasodium Glutamate Diacetate, Silica, Tocopherol, Sea Salt, Potassium Sorbate, Sodium Benzoate, Sodium Hydroxide, Citric Acid, Benzoic Acid, Aminomethyl Propanol, Phenoxyethanol.
- Questions or comments
- PRINCIPAL DISPLAY PANEL - 15 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
AGE BRIGHT SPOT FADER
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68479-806 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Butylene Glycol (UNII: 3XUS85K0RA) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) Isopropyl Lauroyl Sarcosinate (UNII: LYR06W430J) Niacinamide (UNII: 25X51I8RD4) Propanediol (UNII: 5965N8W85T) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) Glycereth-26 (UNII: NNE56F2N14) Saccharide Isomerate (UNII: W8K377W98I) Hexylresorcinol (UNII: R9QTB5E82N) CLARY SAGE OIL (UNII: 87L0D4U3M0) Malic Acid (UNII: 817L1N4CKP) ALOE VERA LEAF (UNII: ZY81Z83H0X) LAVENDER OIL (UNII: ZBP1YXW0H8) LEMON OIL (UNII: I9GRO824LL) ROSEMARY OIL (UNII: 8LGU7VM393) LAVANDIN OIL (UNII: 9RES347CKG) BETASIZOFIRAN (UNII: 2X51AD1X3T) CARRAGEENAN SODIUM (UNII: 7CY8BVL34N) Thymol (UNII: 3J50XA376E) Terpineol (UNII: R53Q4ZWC99) Pullulan (UNII: 8ZQ0AYU1TT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Glycerin (UNII: PDC6A3C0OX) Dimethyl Isosorbide (UNII: SA6A6V432S) PEG-12 Dimethicone/PPG-20 Crosspolymer (UNII: 965K72OQXO) Xanthan Gum (UNII: TTV12P4NEE) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Ethylhexylglycerin (UNII: 147D247K3P) Tetrasodium Glutamate Diacetate (UNII: 5EHL50I4MY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Tocopherol (UNII: R0ZB2556P8) Sea Salt (UNII: 87GE52P74G) Potassium Sorbate (UNII: 1VPU26JZZ4) Sodium Benzoate (UNII: OJ245FE5EU) Sodium Hydroxide (UNII: 55X04QC32I) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Benzoic Acid (UNII: 8SKN0B0MIM) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) Phenoxyethanol (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68479-806-02 1 in 1 CARTON 06/06/2019 1 15 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:68479-806-00 2 mL in 1 POUCH; Type 0: Not a Combination Product 06/06/2019 3 NDC:68479-806-01 6 mL in 1 TUBE; Type 0: Not a Combination Product 06/06/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 06/06/2019 Labeler - Dermalogica, Inc (177698560) Establishment Name Address ID/FEI Business Operations McKenna Labs, Inc. 090631412 MANUFACTURE(68479-806)