Label: CEO-TWO- laxative suppository
- NDC Code(s): 0283-1023-00, 0283-1023-02, 0283-1023-36, 0283-1023-54
- Packager: Beutlich Pharmaceuticals, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
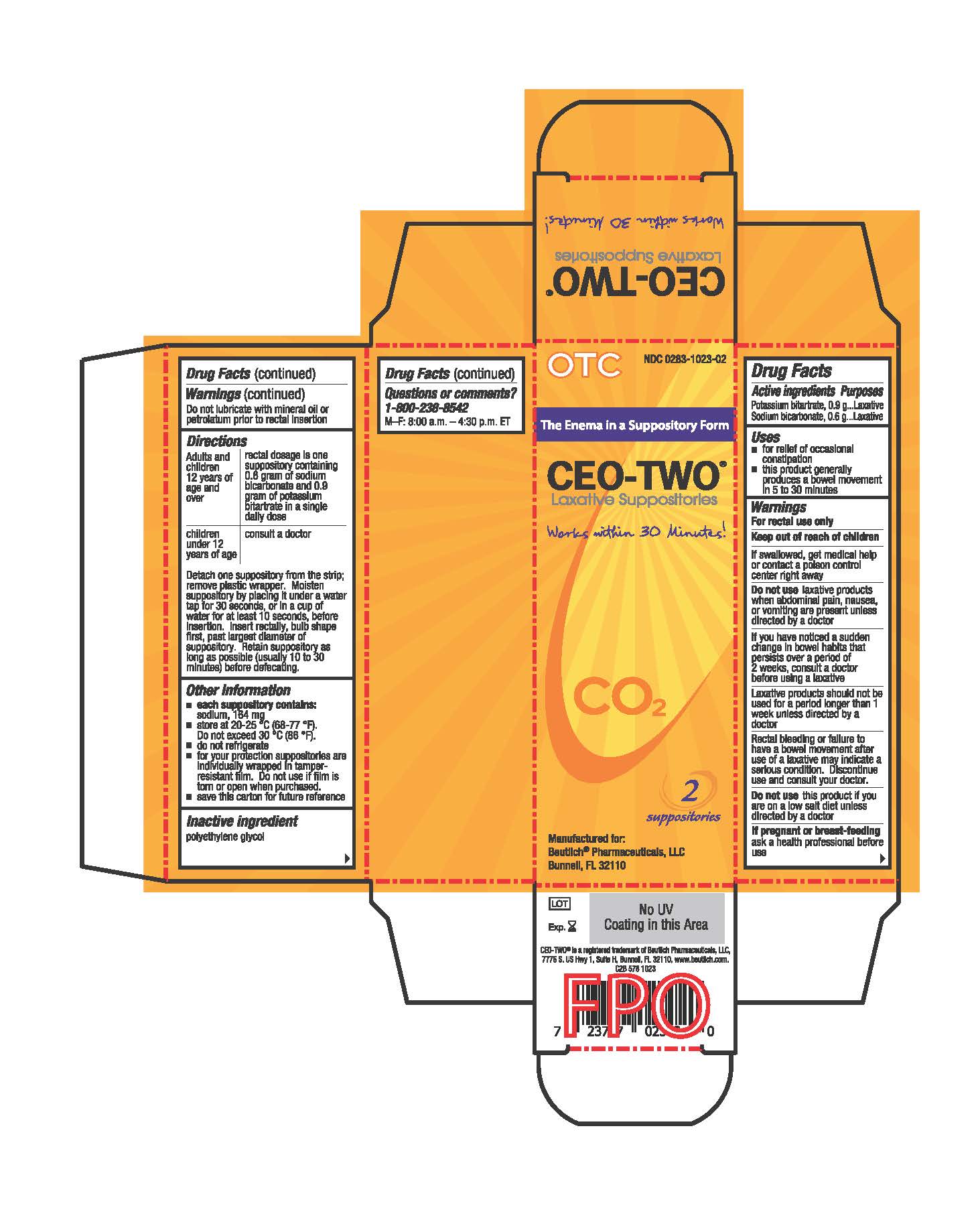
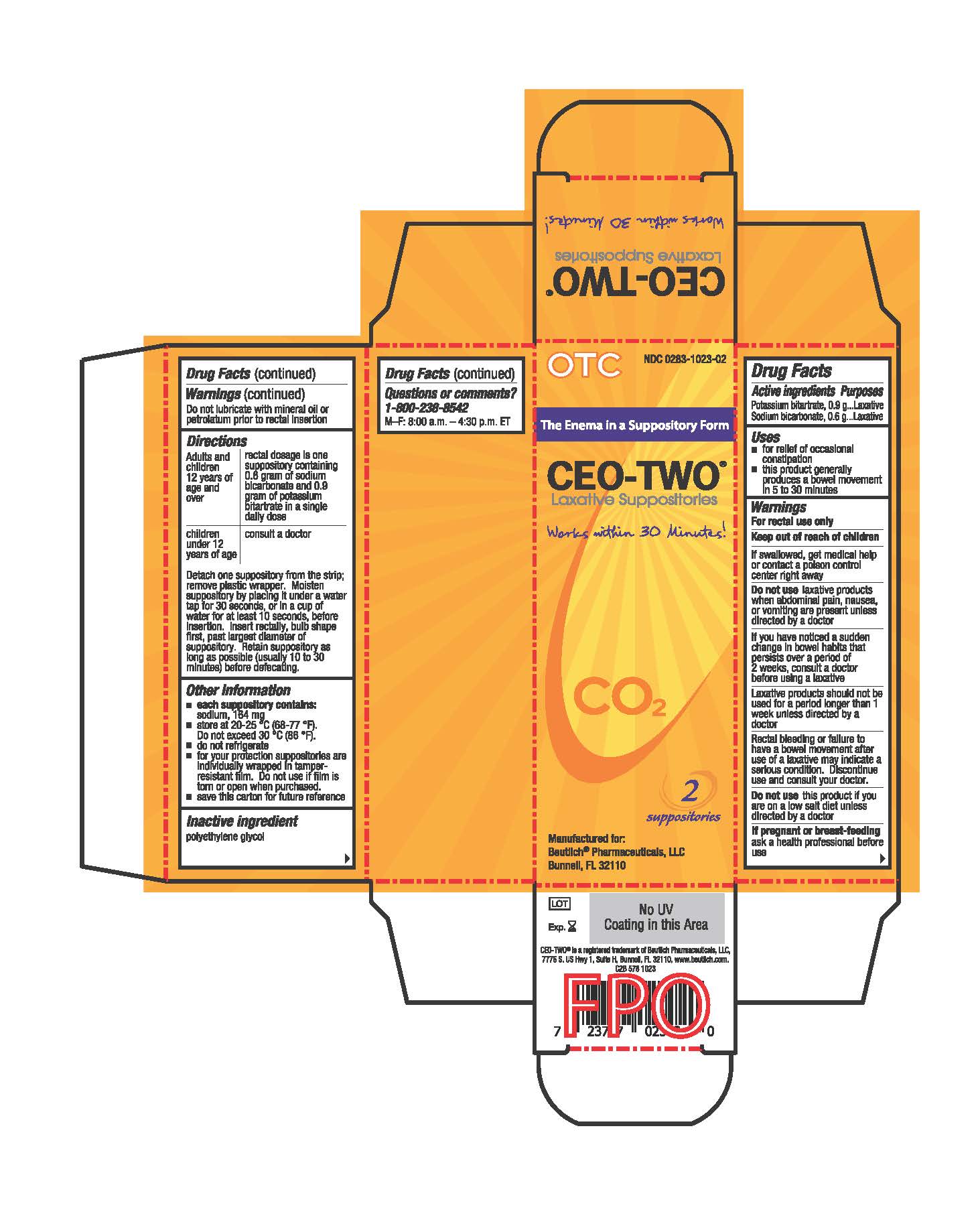
- Active ingredients
- Purposes
- Uses
- Warnings
- Keep out of reach of children
- DO NOT USE
- ASK DOCTOR
- STOP USE
- If pregnant or breast-feeding
-
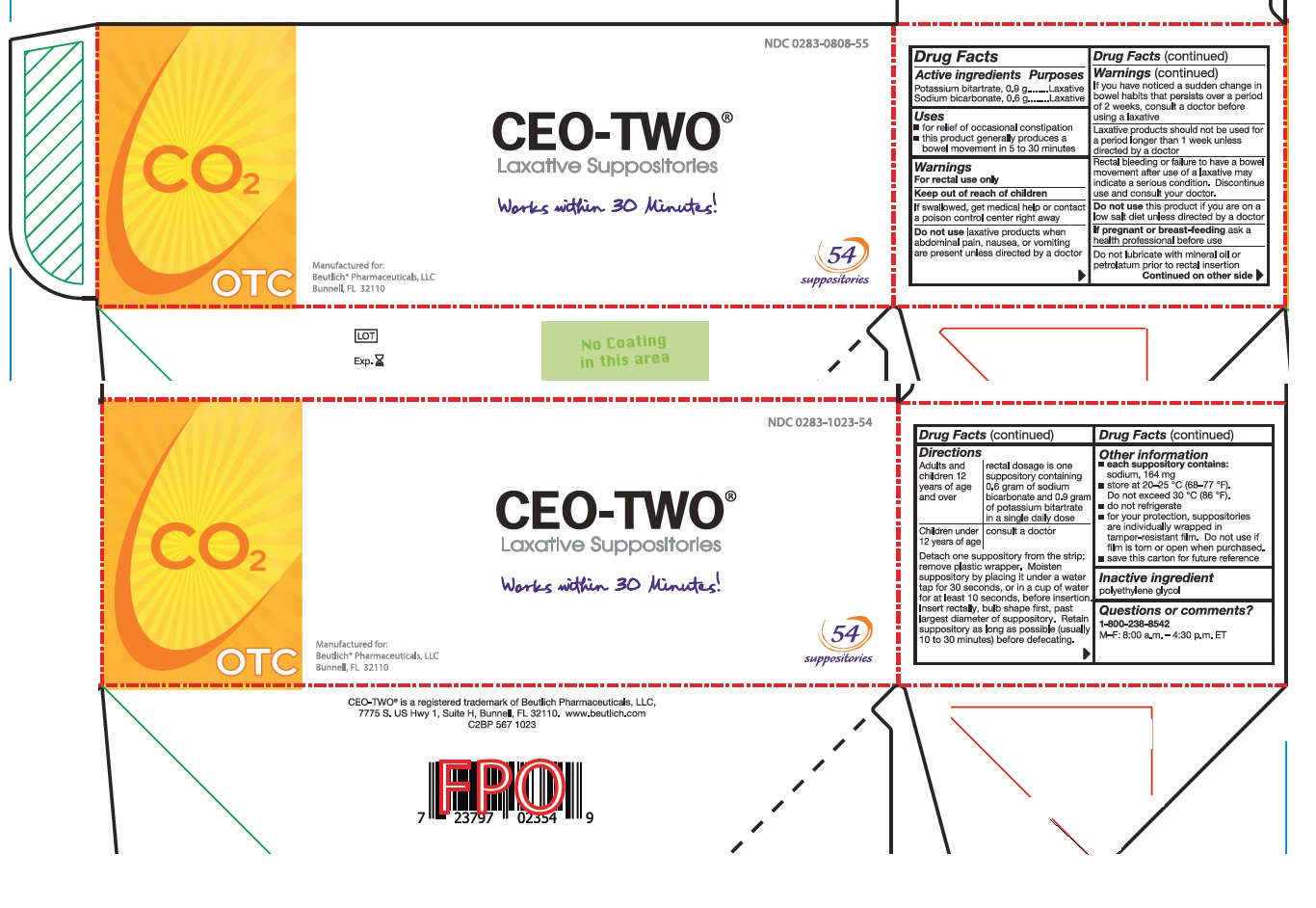
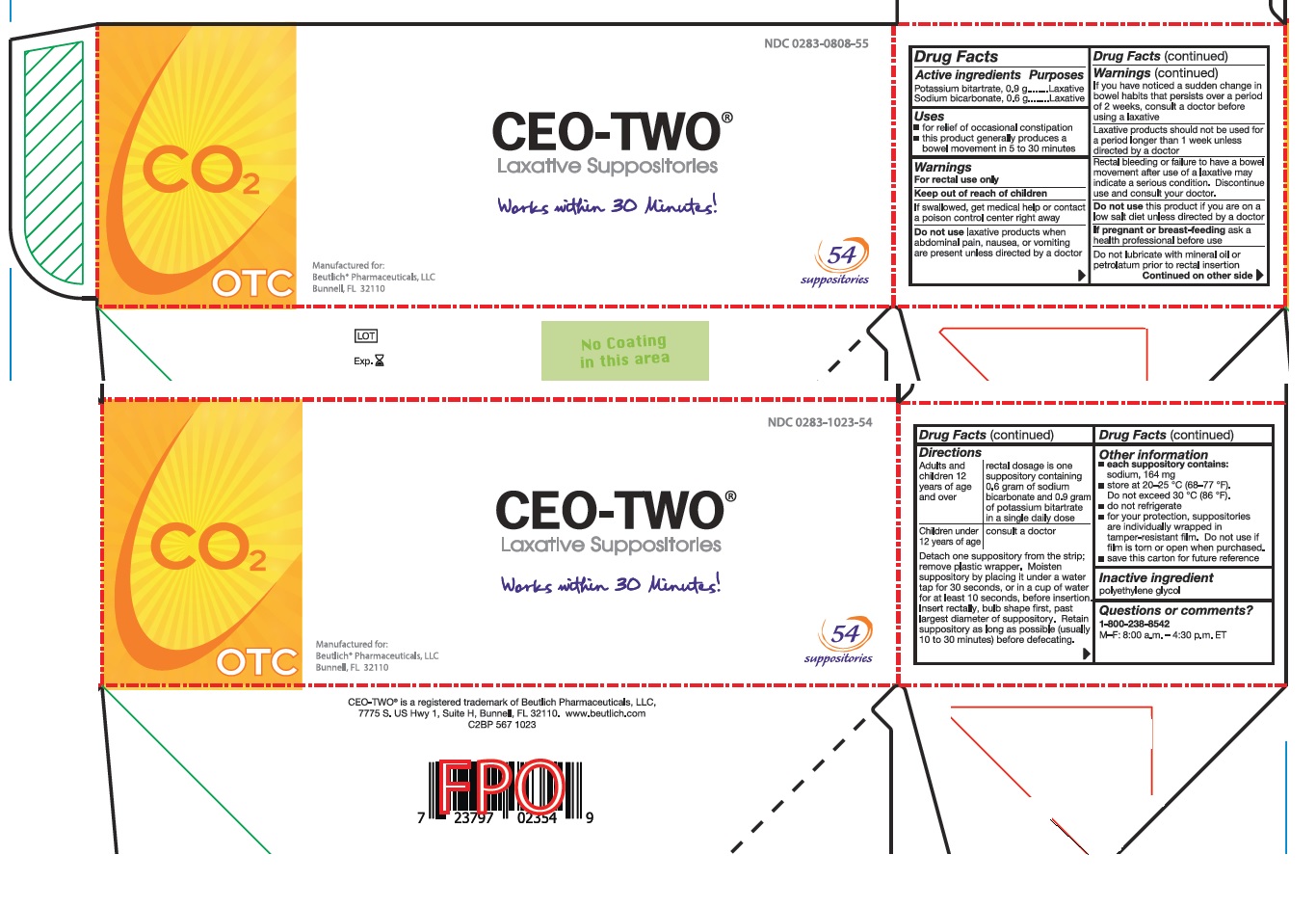
Directions
Adults and children 12 years of age and over: rectal dosage is one suppository containing 0.6 gram of sodium bicarbonate and 0.9 gram of potassium bitartrate in a single daily dose
Children under 12 years of age: consult a doctor
Detach one suppository from the strip; remove plastic wrapper. Moisten suppository by placing it under a water tap for 30 seconds, or in a cup of water for at least 10 seconds, before insertion. Insert rectally, bulb shape first, past largest diameter of suppository. Retain suppository as long as possible (usually 10 to 30 minutes) before defecating.
- INFORMATION FOR PATIENTS
- Other information
- Inactive ingredient
- Questions or comments?
- Prinicipal Display Panel - 54 count
-
INGREDIENTS AND APPEARANCE
CEO-TWO
laxative suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0283-1023 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM BITARTRATE (UNII: NPT6P8P3UU) (CARBON DIOXIDE - UNII:142M471B3J) CARBON DIOXIDE 0.9 g in 5.7 g SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 0.6 g in 5.7 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 1450 (UNII: OJ4Z5Z32L4) 4.2 g in 5.7 g Product Characteristics Color white Score Shape BULLET Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0283-1023-02 2 in 1 BOX 10/15/2023 1 NDC:0283-1023-00 5.7 g in 1 DOSE PACK; Type 0: Not a Combination Product 2 NDC:0283-1023-36 6 in 1 BOX 10/15/2023 2 NDC:0283-1023-00 5.7 g in 1 DOSE PACK; Type 0: Not a Combination Product 3 NDC:0283-1023-54 54 in 1 BOX 10/15/2023 3 NDC:0283-1023-00 5.7 g in 1 DOSE PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 10/15/2023 Labeler - Beutlich Pharmaceuticals, LLC (005209325) Registrant - Beutlich Pharmaceuticals, LLC (005209325) Establishment Name Address ID/FEI Business Operations Beutlich Pharmaceuticals, LLC 005209325 label(0283-1023) , pack(0283-1023) Establishment Name Address ID/FEI Business Operations Speciality Pharma Manufacturing 013957125 manufacture(0283-1023)