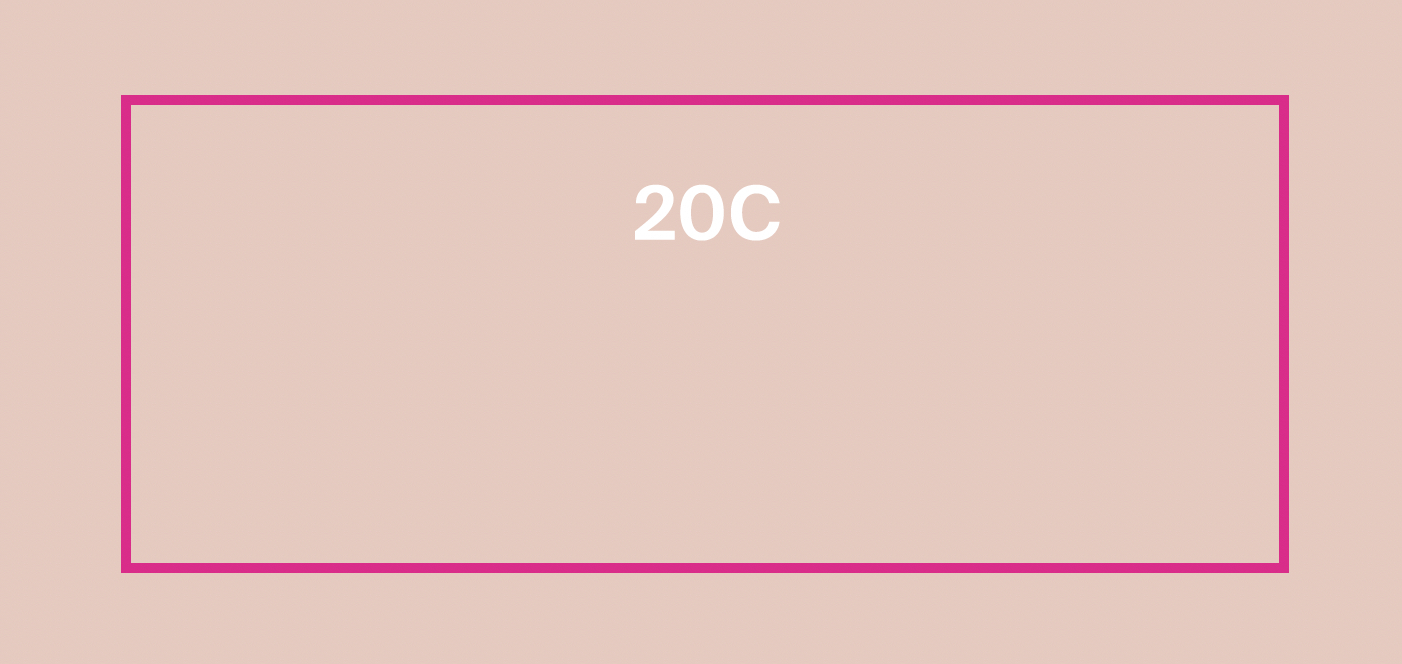
Label: PROTECTINT 20C- homosalate, octisalate, zinc oxide cream
PROTECTINT 26W- homosalate, octisalate, zinc oxide cream
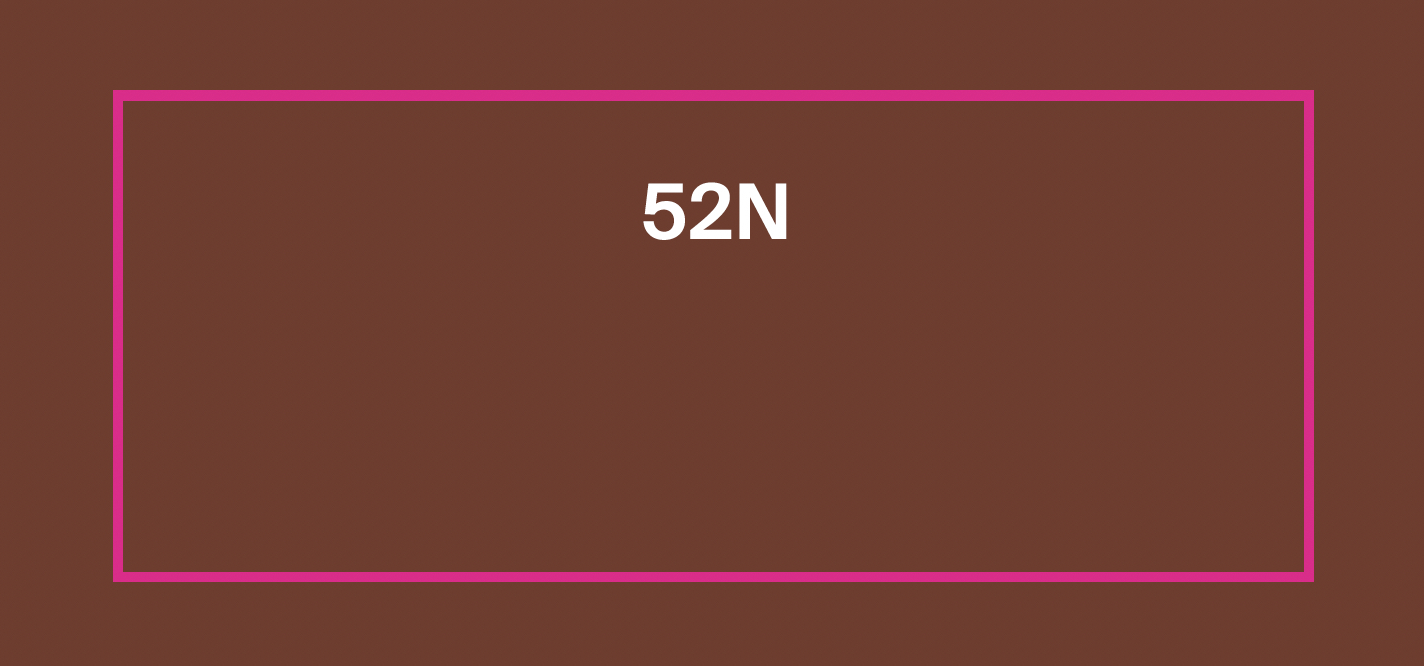
PROTECTINT 32N- homosalate, octisalate, zinc oxide cream
PROTECTINT 34C- homosalate, octisalate, zinc oxide cream
PROTECTINT 58W- homosalate, octisalate, zinc oxide cream
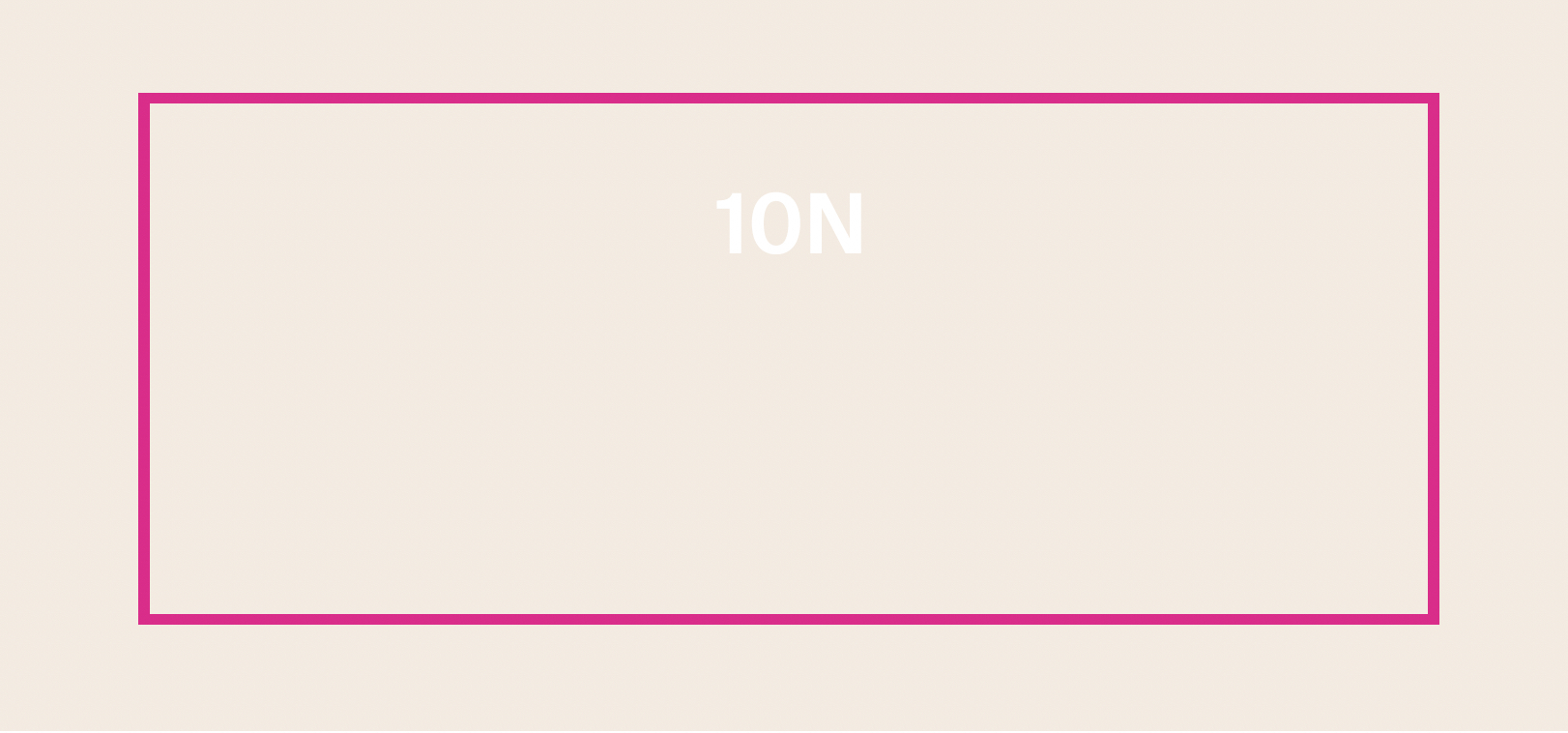
PROTECTINT 10N- homosalate, octisalate, zinc oxide cream
PROTECTINT 24N- homosalate, octisalate, zinc oxide cream
PROTECTINT 22W- homosalate, octisalate, zinc oxide cream
PROTECTINT 30W- homosalate, octisalate, zinc oxide cream
PROTECTINT 46N- homosalate, octisalate, zinc oxide cream
PROTECTINT 42C- homosalate, octisalate, zinc oxide cream
PROTECTINT 52N- homosalate, octisalate, zinc oxide cream
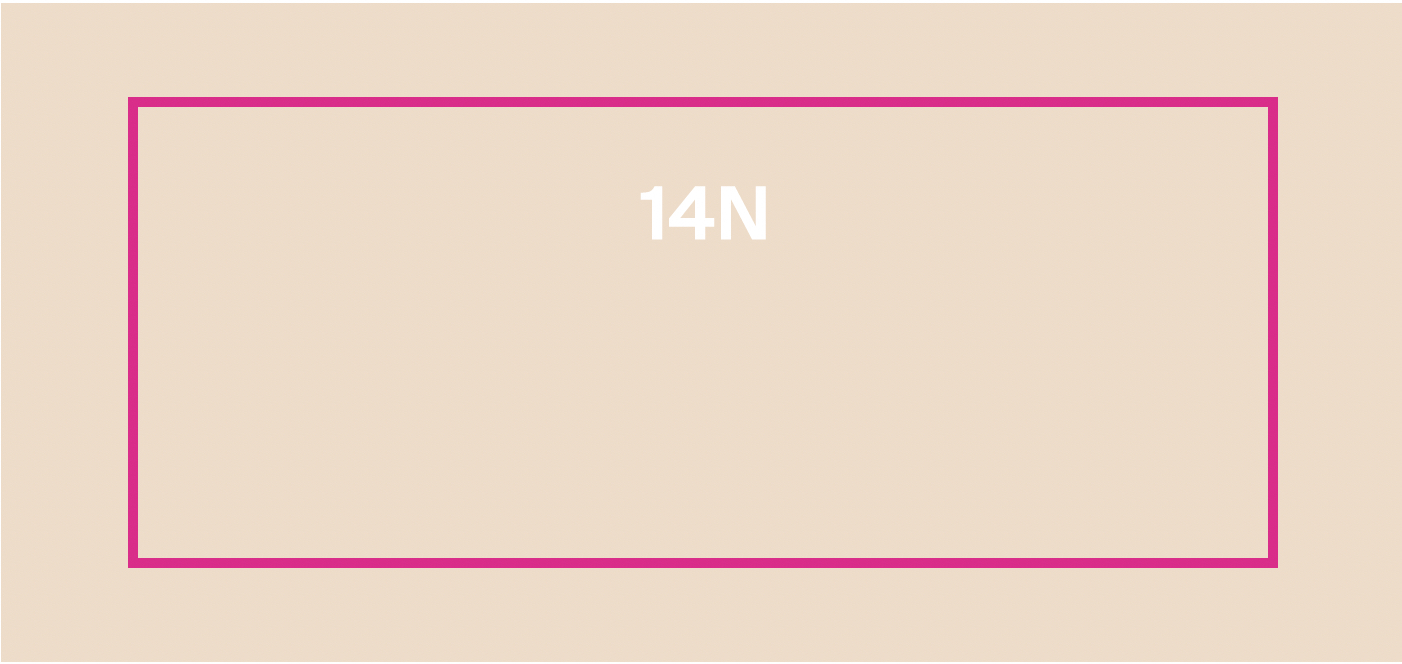
PROTECTINT 14N- homosalate, octisalate, zinc oxide cream
PROTECTINT 40W- homosalate, octisalate, zinc oxide cream
-
NDC Code(s):
75936-622-01,
75936-622-02,
75936-624-01,
75936-624-02, view more75936-625-01, 75936-625-02, 75936-626-01, 75936-626-02, 75936-627-01, 75936-627-02, 75936-628-01, 75936-628-02, 75936-629-01, 75936-629-02, 75936-630-01, 75936-630-02, 75936-631-01, 75936-631-02, 75936-632-01, 75936-632-02, 75936-633-01, 75936-633-02, 75936-634-01, 75936-634-02, 75936-635-01, 75936-635-02, 75936-636-01, 75936-636-02
- Packager: Supergoop, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Directions
Apply generously and evenly 15 minutes before sun exposure
Reapply at least every 2 hours
Use a water resistant sunscreen if swimming or sweating
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.- 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
-
INACTIVE INGREDIENT
Inactive Ingredients:IAqua/Water/Eau, Isononyl Isononanoate, Butyloctyl Salicylate, Propanediol, Silica, Glycerin,1,2-Hexanediol, Lecithin, Coco-Caprylate/Caprate, Cetearyl Alcohol, Lauryl Glucoside, Polyglyceryl-2 Dipolyhydroxystearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Ectoin, Coco-Glucoside, Polyhydroxystearic acid, Citric Acid, Triethoxycaprylylsilane, Morinda Citrifolia Extract, Xanthan Gum, Sodium Phytate, Bentonite, Sodium Hyaluronate, Tocopherol
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROTECTINT 20C
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-625 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color white (20C) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-625-02 1 in 1 BOX 11/22/2023 1 NDC:75936-625-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 26W
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-628 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color white (26W) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-628-02 1 in 1 BOX 11/22/2023 1 NDC:75936-628-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 32N
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-630 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color brown (32N) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-630-02 1 in 1 BOX 11/22/2023 1 NDC:75936-630-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 34C
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-631 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color brown (34C) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-631-02 1 in 1 BOX 11/22/2023 1 NDC:75936-631-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 58W
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-636 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color brown (58W) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-636-02 1 in 1 BOX 11/22/2023 1 NDC:75936-636-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 10N
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-622 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) PHYTATE SODIUM (UNII: 88496G1ERL) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) Product Characteristics Color white (10N) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-622-02 1 in 1 BOX 11/22/2023 1 NDC:75936-622-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 24N
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-627 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color white (24N) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-627-02 1 in 1 BOX 11/22/2023 1 NDC:75936-627-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 22W
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-626 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color white (22W) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-626-02 1 in 1 BOX 11/22/2023 1 NDC:75936-626-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 30W
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-629 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color brown (30W) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-629-02 1 in 1 BOX 11/22/2023 1 NDC:75936-629-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 46N
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-634 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color brown (46N) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-634-02 1 in 1 BOX 11/22/2023 1 NDC:75936-634-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 42C
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-633 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color brown (42C) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-633-02 1 in 1 BOX 11/22/2023 1 NDC:75936-633-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 52N
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-635 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color brown (52N) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-635-02 1 in 1 BOX 11/22/2023 1 NDC:75936-635-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 14N
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-624 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color white (14N) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-624-02 1 in 1 BOX 11/22/2023 1 NDC:75936-624-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 PROTECTINT 40W
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-632 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.58 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) NONI FRUIT (UNII: 7829X3G2X5) PHYTATE SODIUM (UNII: 88496G1ERL) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPANEDIOL (UNII: 5965N8W85T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ECTOINE (UNII: 7GXZ3858RY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color brown (40W) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-632-02 1 in 1 BOX 11/22/2023 1 NDC:75936-632-01 35 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/22/2023 Labeler - Supergoop, LLC (117061743)