Label: BUILDABLE BLUR- homosalate, octisalate, zinc oxide cream
- NDC Code(s): 68577-134-01
- Packager: COSMAX USA, CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
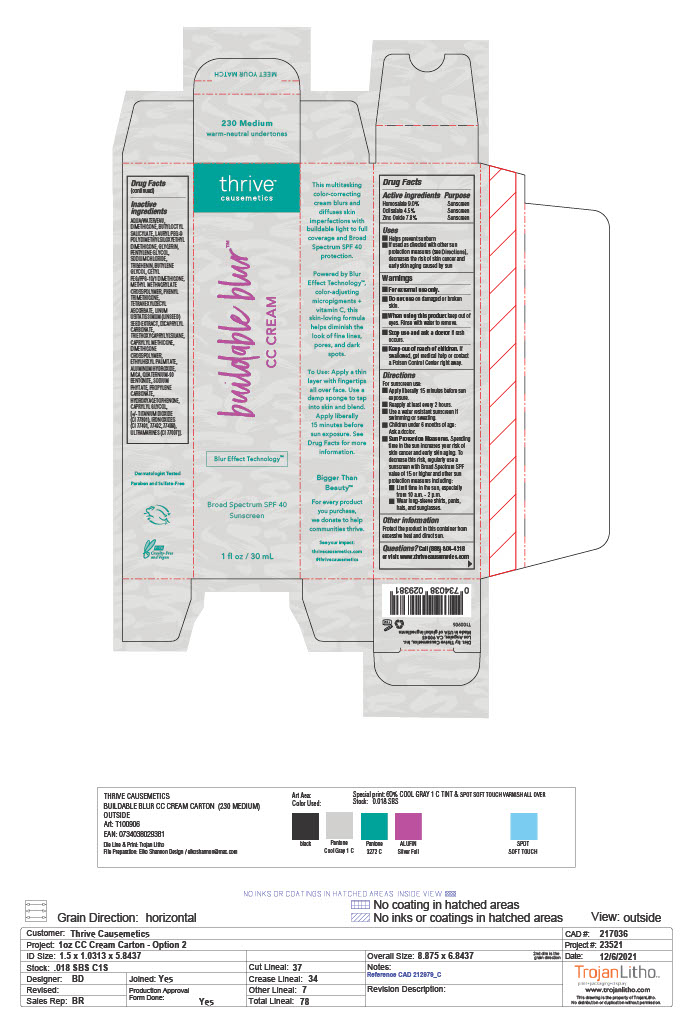
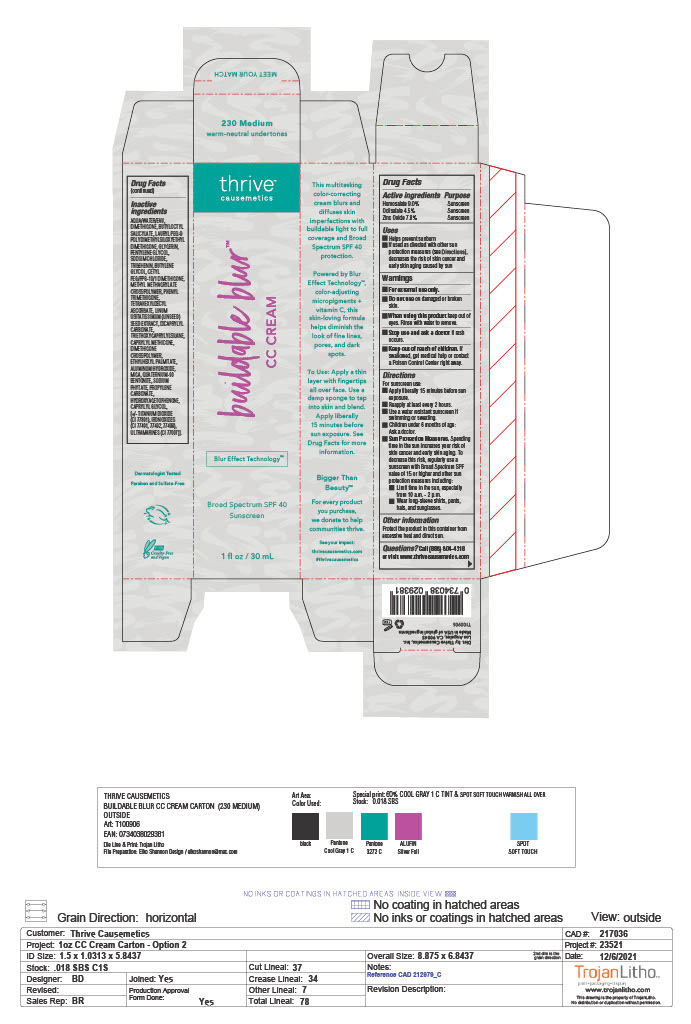
- ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNINGS
-
DIRECTIONS
Directions
For sunscreen use:
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months of age: Ask a doctor
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
-Limit time in the sun, especially from 10 a.m. - 2 p.m.
-Wear long-sleeve shirts, pants, hats, and sunglasses.
- OTHER INFORMATION
- QUESTIONS
-
INACTIVE INGREDIENT
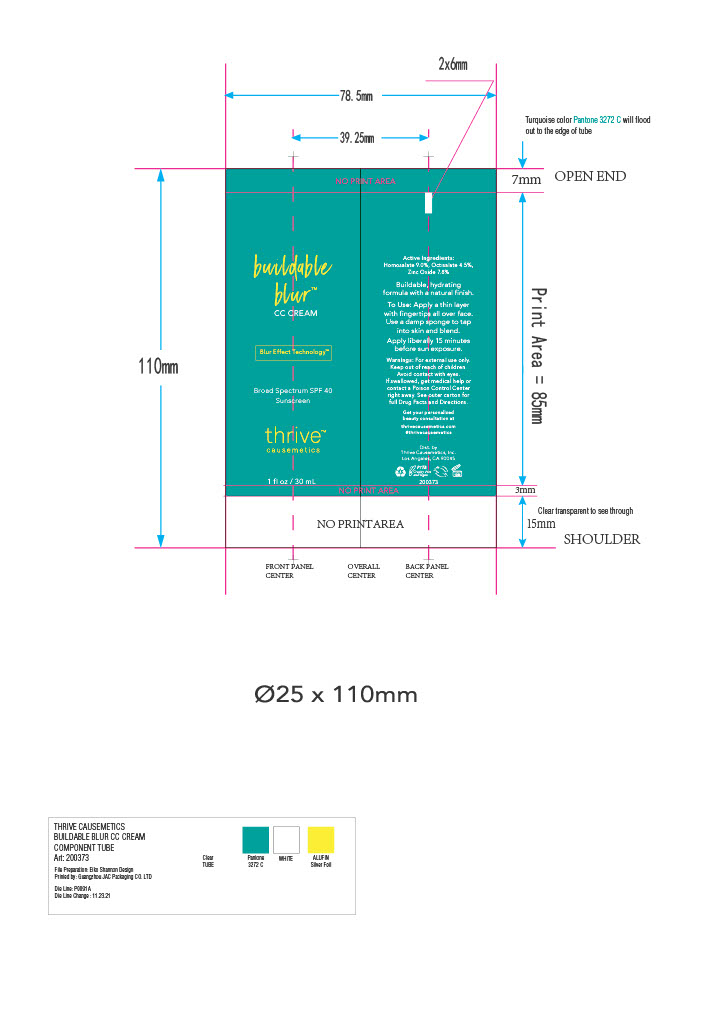
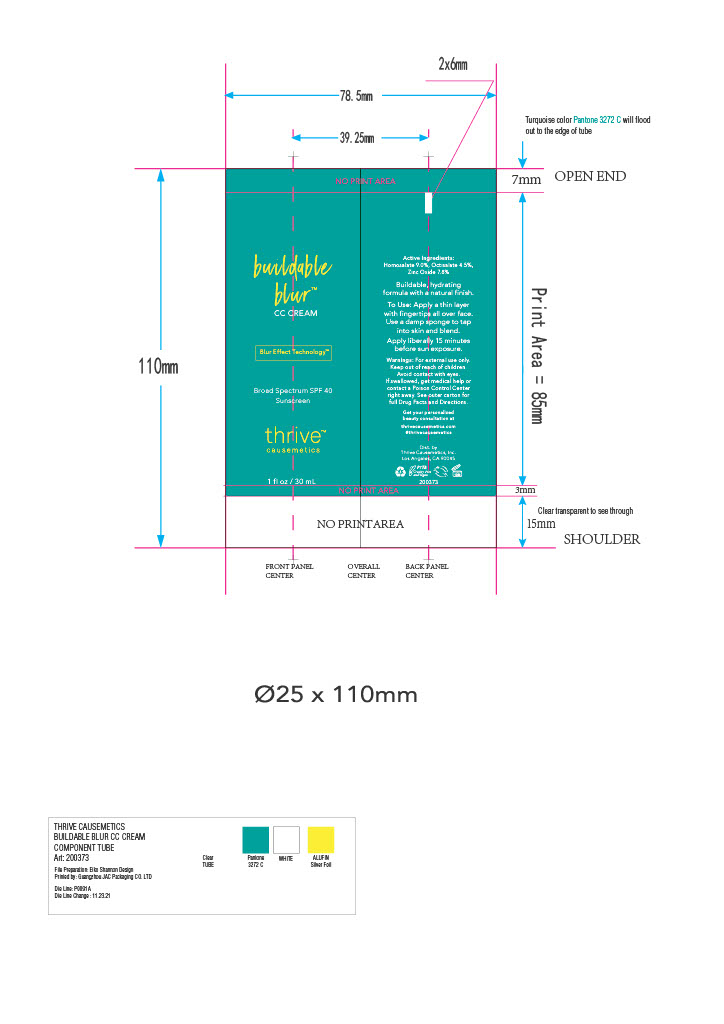
Inactive ingredients
AQUA/WATER/EAU, DIMETHICONE, BUTYLOCTYL SALICYLATE, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, GLYCERIN, PENTYLENE GLYCOL, SODIUM CHLORIDE, TRIBEHENIN, BUTYLENE GLYCOL, CETYL PEG/PPG-10/1 DIMETHICONE, METHYL METHACRYLATE CROSSPOLYMER, PHENYL TRIMETHICONE, TETRAHEXYLDECYL ASCORBATE, LINUM USITATISSIMUM (LINSEED) SEED EXTRACT, DICAPRYLYL CARBONATE, TRIETHOXYCAPRYLYLSILANE, CAPRYLYL METHICONE, DIMETHICONE CROSSPOLYMER, ETHYLHEXYL PALMITATE, ALUMINUM HYDROXIDE, MICA, QUATERNIUM-90 BENTONITE, SODIUM PHYTATE, PROPYLENE CARBONATE, HYDROXYACETOPHENONE, CAPRYLYL GLYCOL, [+/- TITANIUM DIOXIDE (CI 77891), IRON OXIDES (CI 77491, 77492, 77499), ULTRAMARINES (CI 77007)].
- Primary Package
- Secondary Package
-
INGREDIENTS AND APPEARANCE
BUILDABLE BLUR
homosalate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68577-134 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 mg in 100 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 7.8 mg in 100 mg OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 mg in 100 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) FLAX SEED (UNII: 4110YT348C) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MICA (UNII: V8A1AW0880) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) DIMETHICONE (UNII: 92RU3N3Y1O) TRIBEHENIN (UNII: 8OC9U7TQZ0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) PHYTATE SODIUM (UNII: 88496G1ERL) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PENTYLENE GLYCOL (UNII: 50C1307PZG) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PROPYLENE CARBONATE (UNII: 8D08K3S51E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) ULTRAMARINE BLUE (UNII: I39WR998BI) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68577-134-01 1 in 1 CARTON 12/01/2022 1 30 mg in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2022 Labeler - COSMAX USA, CORPORATION (010990210) Establishment Name Address ID/FEI Business Operations COSMAX USA. CORPORATION 010990210 manufacture(68577-134)