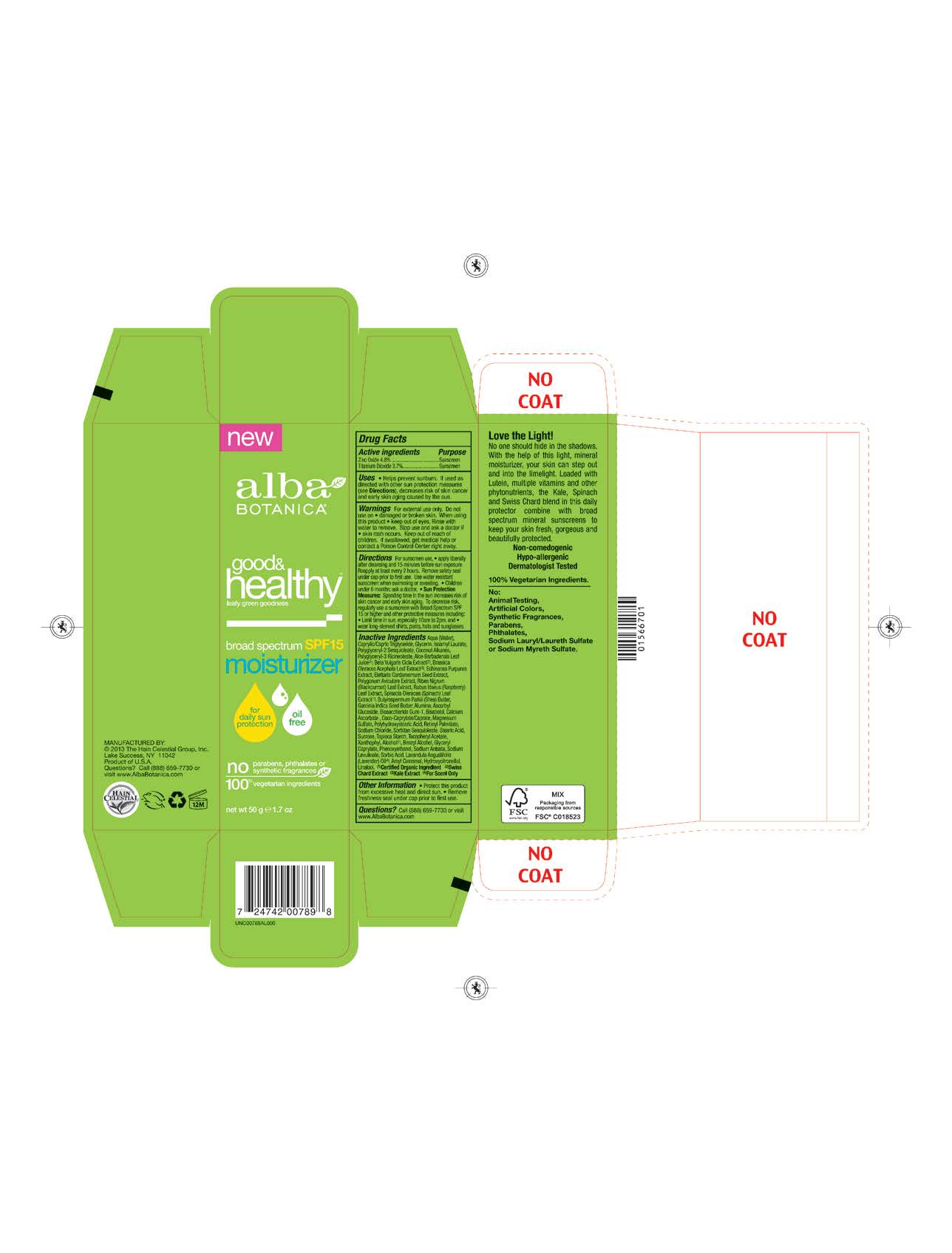
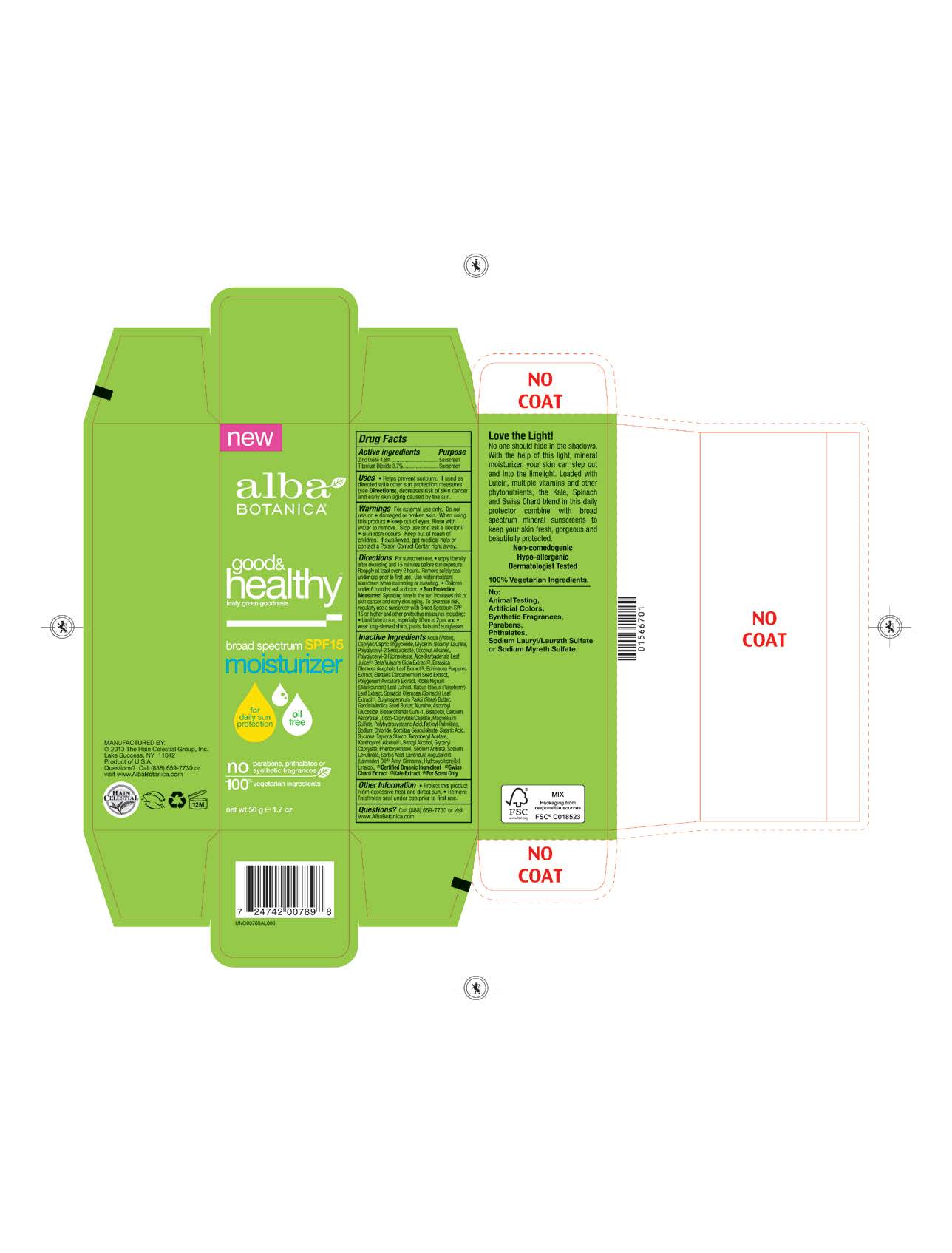
Label: ALBA GOOD AND HEALTHY MOISTURIZER SPF15- zinc oxide, titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 61995-2789-2 - Packager: The Hain Celestial Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 29, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
Water, Caprylic Capric Triglyceride,Glycerin, Isoamyl Laurate, Coconut Alkanes, Polyglyceryl-2 Sesquioleate, Polyglyceryl-3 Ricinoleate, Aloe Barbadensis Leaf Juice(1), Polyhydroxystearic Acid, Echinacea Purpurea Extract, Polygonum Aviculare Extract, Brassica Oleracea Acephala Leaf Extract, Elettaria Cardamomum Seed Extract, Ribes Nigrum (Blackcurrant) Leaf Extract, Rubus Idaeus (Raspberry) Leaf Extract, Spinacia Oleracea (Spinach) Leaf Extract, Garcinia Indica Seed Butter, Ascorbyl Glucoside, Biosaccharide Gum-1, Bisabolol, Retinyl Palmitate,Sucrose, Tapioca Starch,Lavandula Angusiofolia (Lavender) Oil, Tocopheryl Acetate, Butyrospermum Parkii (Shea) Butter, Beta Vulgaris (Beet)Root Extract, Alumina, Calcium Ascorbate, Coco-Caprylate/Caprate ,Xanthophyl,Glyceryl Caprylate, Magnesium Sulfate, Sodium Chloride, Sorbitan Sesquioleate, Stearic Acid, Alcohol (1), Benzyl Alcohol, Phenoxyethanol, Sodium Anisate, Sodium Lavulinate.
(1) Certified Organicf Ingredient
-
INDICATIONS & USAGE
Helps prevents sunburns. If uses as directed with other sun protection measures, decreases risk of skin cancer and early skin aging caused by sun exposure. Skin Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease risk, regularly use sunscreen with Broad Spectrum SPF 15 or higher and other protective measures including: limit time in sun, especially from10am to 2pm, and wear long sleeved shirts, pants, hats and sunglases.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALBA GOOD AND HEALTHY MOISTURIZER SPF15
zinc oxide, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61995-2789 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 3.7 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 4.8 g in 100 g Inactive Ingredients Ingredient Name Strength RUBUS IDAEUS LEAF (UNII: 8O2V33JG64) RIBES NIGRUM LEAF (UNII: Z46FSZ2M25) STARCH, TAPIOCA (UNII: 24SC3U704I) CARDAMOM (UNII: 8BC4CUT4JL) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) LEVOMENOL (UNII: 24WE03BX2T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SUCROSE (UNII: C151H8M554) LUTEIN (UNII: X72A60C9MT) BETA VULGARIS (UNII: 4G174V5051) CALCIUM ASCORBATE (UNII: 183E4W213W) ALCOHOL (UNII: 3K9958V90M) BENZYL ALCOHOL (UNII: LKG8494WBH) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALOE VERA LEAF (UNII: ZY81Z83H0X) ISOAMYL LAURATE (UNII: M1SLX00M3M) COCONUT ALKANES (UNII: 1E5KJY107T) GLYCERIN (UNII: PDC6A3C0OX) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) WATER (UNII: 059QF0KO0R) SHEA BUTTER (UNII: K49155WL9Y) ALUMINUM OXIDE (UNII: LMI26O6933) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) MAGNESIUM SULFATE (UNII: DE08037SAB) SODIUM CHLORIDE (UNII: 451W47IQ8X) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) STEARIC ACID (UNII: 4ELV7Z65AP) ECHINACEA PURPUREA ROOT (UNII: OS64WTR4KU) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GARCINIA INDICA SEED BUTTER (UNII: US2H3D7800) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) SORBIC ACID (UNII: X045WJ989B) KALE (UNII: 0Y3L4J38H1) SODIUM ANISATE (UNII: F9WFJ28MV9) SODIUM LEVULINATE (UNII: VK44E1MQU8) LAVENDER OIL (UNII: ZBP1YXW0H8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61995-2789-2 1 in 1 CARTON 01/27/2014 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/27/2014 Labeler - The Hain Celestial Group, Inc. (858894996) Registrant - The Hain Celestial Group, Inc. (858894996) Establishment Name Address ID/FEI Business Operations The Hain Celestial Group, Inc. 858894996 manufacture(61995-2789)