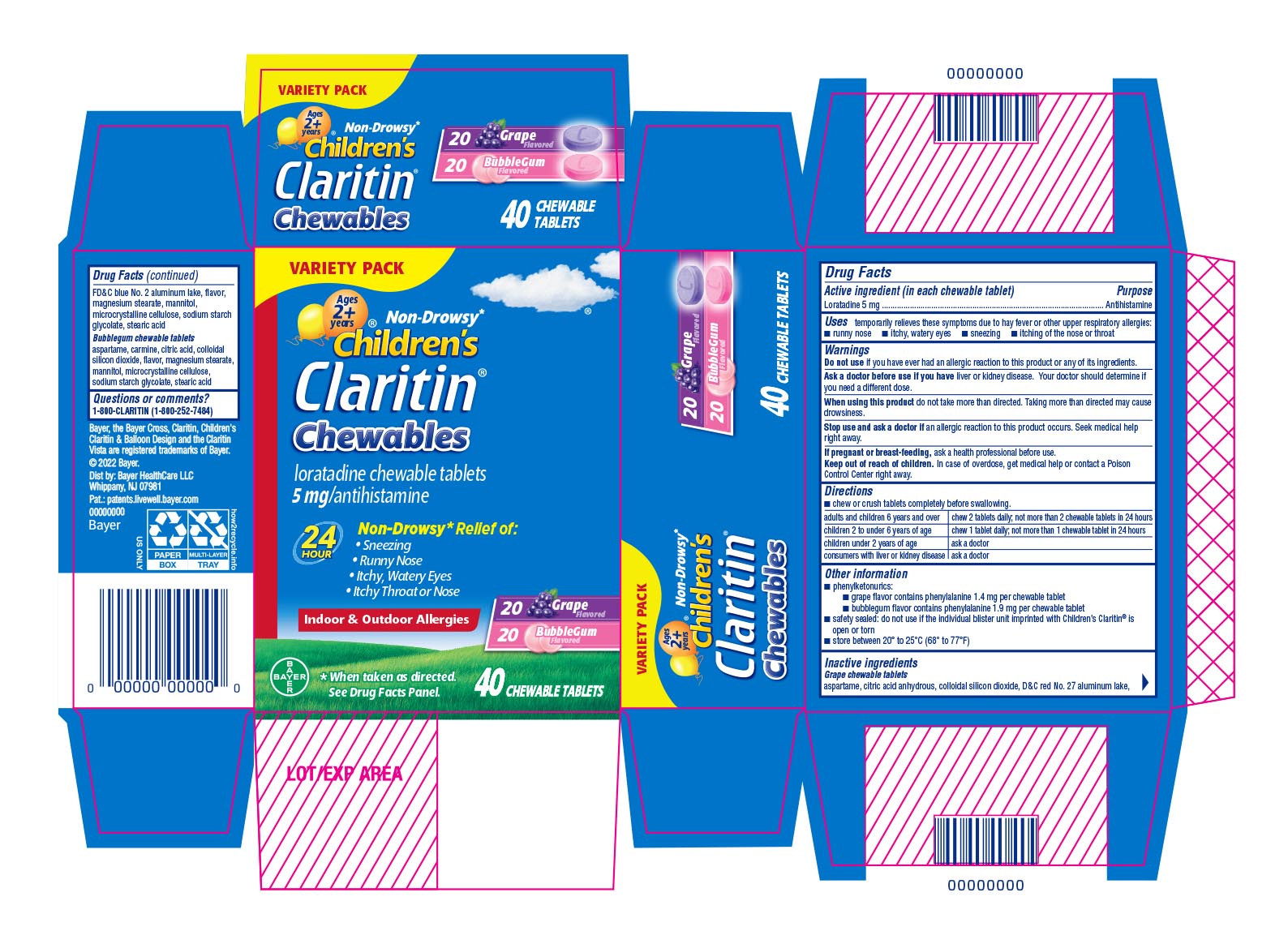
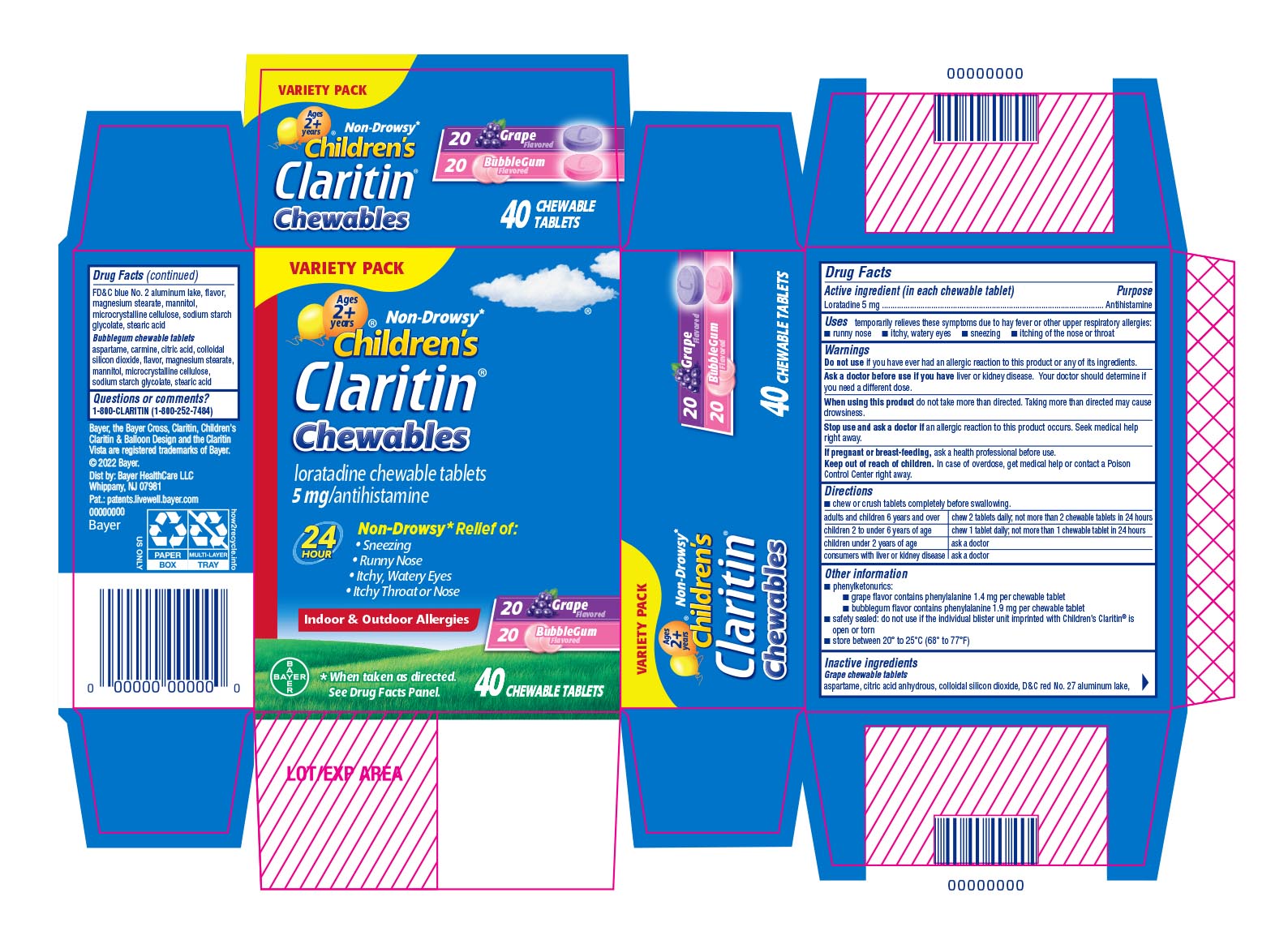
Label: CLARITIN CHEWABLE GRAPE AND BUBBLEGUM- loratadine kit
- NDC Code(s): 11523-0123-1
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
-
Directions
- chew or crush tablets completely before swallowing.
adults and children 6 years and over chew 2 tablets daily; not more than 2 chewable tablets in 24 hours children 2 to under 6 years of age chew 1 tablet daily; not more than 1 chewable tablet in 24 hours children under 2 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other Information
-
Inactive ingredients
Grape chewable tablets
aspartame, citric acid anhydrous, colloidal silicon dioxide, D&C red No. 27 aluminum lake, FD&C blue No. 2 aluminum lake, flavor, magnesium stearate, mannitol, microcrystalline cellulose, sodium starch glycolate, stearic aicd
Bubblegum chewable tablets
aspartame, carmine, citric acid, colloidal silicon dioxide, flavor, magnesium stearate, mannitol, microcrystalline cellulose, sodium starch glycolate, stearic acid
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL - 40 Chewable Tablet Blister Pack Carton
VARIETY PACK
ages
2 +years
Non-Drowsy*Children's
Claritin ®Chewables
loratadine chewable tablets
5 mg/antihistamine
24 HourNon-Drowsy* Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
Indoor & Outdoor Allergies
20 Grape Flavored
20 Bubblegum Flavored
*When taken as directed. See Drug Facts Panel.
40 CHEWABLE TABLETS
-
INGREDIENTS AND APPEARANCE
CLARITIN CHEWABLE GRAPE AND BUBBLEGUM
loratadine kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-0123 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-0123-1 1 in 1 CARTON; Type 0: Not a Combination Product 09/01/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 20 Part 2 20 Part 1 of 2 CLARITIN
loratadine tablet, chewableProduct Information Item Code (Source) NDC:11523-4330 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 5 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) CARMINIC ACID (UNII: CID8Z8N95N) MANNITOL (UNII: 3OWL53L36A) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color pink (light to medium pink with a slightly and/or mottled appearance) Score no score Shape ROUND (round (flat faced beveled edge)) Size 10mm Flavor BUBBLE GUM Imprint Code C Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021891 09/15/2015 Part 2 of 2 CLARITIN
loratadine tablet, chewableProduct Information Item Code (Source) NDC:11523-4331 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 5 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) MANNITOL (UNII: 3OWL53L36A) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color purple (Light to medium purple with a slightly speckled and/or mottled appearance) Score no score Shape ROUND (flat faced beveled edge) Size 10mm Flavor GRAPE Imprint Code C Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021891 08/23/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021891 08/23/2006 Labeler - Bayer HealthCare LLC. (112117283)