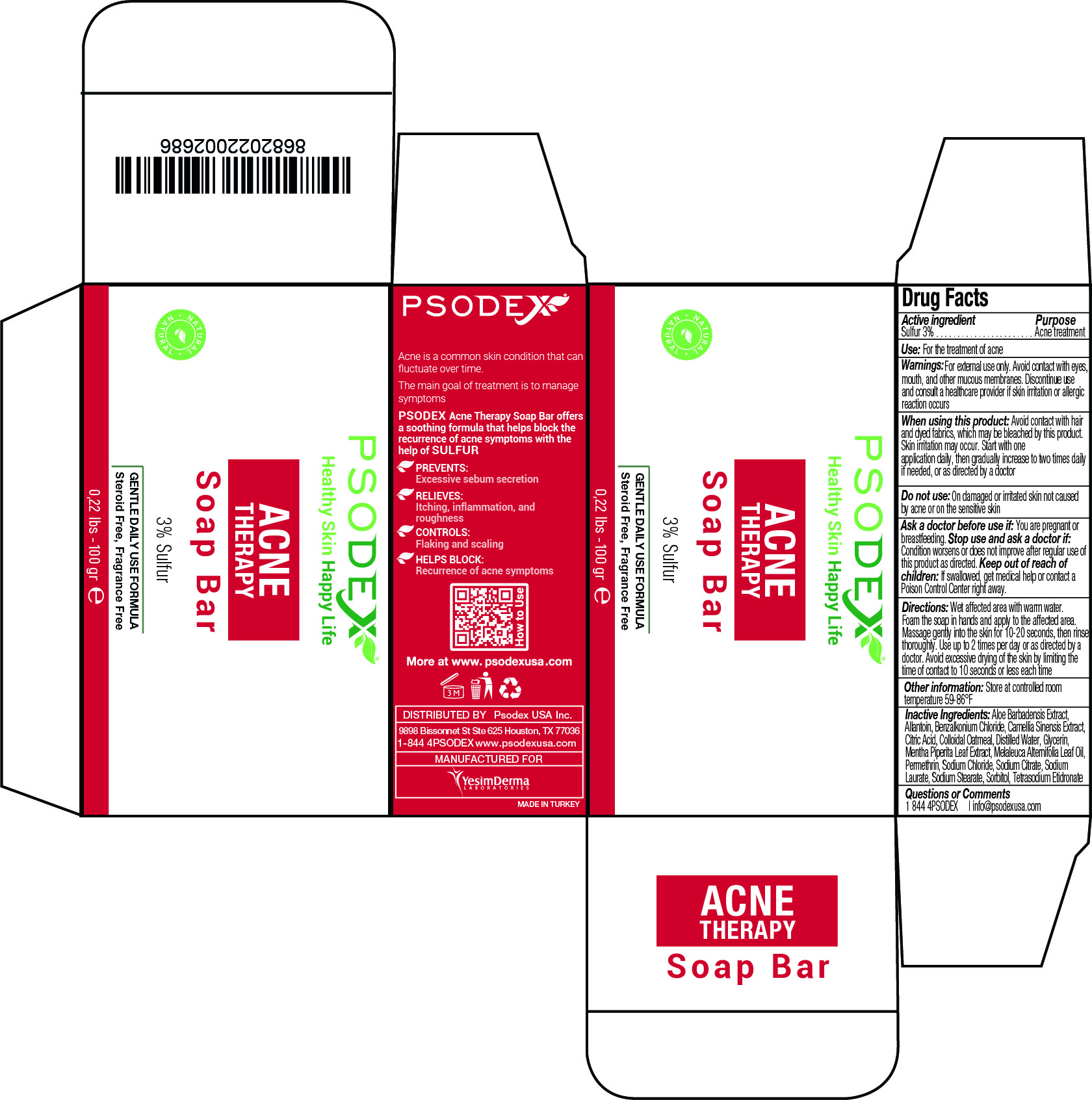
Label: ACNE THERAPY BAR- sulfur soap
- NDC Code(s): 73503-013-00
- Packager: PSODEX USA INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Acne is a common skin condition that can fluctuate over time.
The main goal of treatment is to manage symptoms.
PSODEX Acne Therapy Soap Bar offers a soothing formula that helps block the recurrence of acne symptoms with the help of SULFUR.PREVENTS:
Excessive sebum secretion
RELIEVES:
Itching, inflammation, and roughnessCONTROLS:
Flaking and scalingHELPS BLOCK:
Recurrence of acne symptoms - Active Ingredient / Purpose
- ACTIVE INGREDIENT
- PURPOSE
-
Inactive Ingredients
Aloe Barbadensis Extract,
Allantoin, Benzalkonium Chloride, Camellia Sinensis Extract,
Citric Acid, Colloidal Oatmeal, Distilled Water, Glycerin,
Mentha Piperita Leaf Extract, Melaleuca Alternifolia Leaf Oil,
Permethrin, Sodium Chloride, Sodium Citrate, Sodium
Laurate, Sodium Stearate, Sorbitol, Tetrasodium Etidronate - USE
- INFORMATION FOR OWNERS/CAREGIVERS
-
Directions:
Wet affected area with warm water. Foam the soap in hands and apply to the affected area. Massage gently into the skin for 10-20 seconds, then rinse thoroughly. Use up to 2 times per day or as directed by a doctor. Avoid excessive drying of the skin by limiting the
time of contact to 10 seconds or less each time
- Ask a doctor before use if:
- Ask a doctor before use if:
- Stop use and ask a doctor if:
- Questions or Comments:
- Keep out of reach of children:
- Do not use :
- When using this product :
- Other information :
- Warnings:
- Acne Therapy Soap Bar
-
INGREDIENTS AND APPEARANCE
ACNE THERAPY BAR
sulfur soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73503-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 3 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) 10 g in 100 g TEA LEAF OIL (UNII: VC855RRT77) 5 g in 100 g PERMETHRIN (UNII: 509F88P9SZ) 1 g in 100 g ETIDRONATE TETRASODIUM (UNII: CZZ9T1T1X4) 0.25 g in 100 g ALLANTOIN (UNII: 344S277G0Z) 2 g in 100 g MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL (UNII: VIF565UC2G) 1 g in 100 g SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.25 g in 100 g BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) 0.1 g in 100 g MENTHA PIPERITA LEAF (UNII: A389O33LX6) 7 g in 100 g SODIUM LAURATE (UNII: K146MR5EXO) 10 g in 100 g ALOE VERA LEAF (UNII: ZY81Z83H0X) 7 g in 100 g OATMEAL (UNII: 8PI54V663Y) 2 g in 100 g SODIUM CITRATE (UNII: 1Q73Q2JULR) 0.25 g in 100 g CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.25 g in 100 g WATER (UNII: 059QF0KO0R) 17 mL in 100 g SODIUM STEARATE (UNII: QU7E2XA9TG) 10 g in 100 g GLYCERIN (UNII: PDC6A3C0OX) 23.9 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73503-013-00 1000 g in 1 BOX; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/01/2024 Labeler - PSODEX USA INC (076051073) Registrant - PSODEX USA INC (076051073) Establishment Name Address ID/FEI Business Operations BERKO ILAC VE KIMYA SANAYI ANONIM SIRKETI-SULTANBEYLI SUBESI 533135007 manufacture(73503-013)