Label: ADARA ACNE 911 DRYING- acne drying lotion suspension
- NDC Code(s): 76348-890-01
- Packager: Renu Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
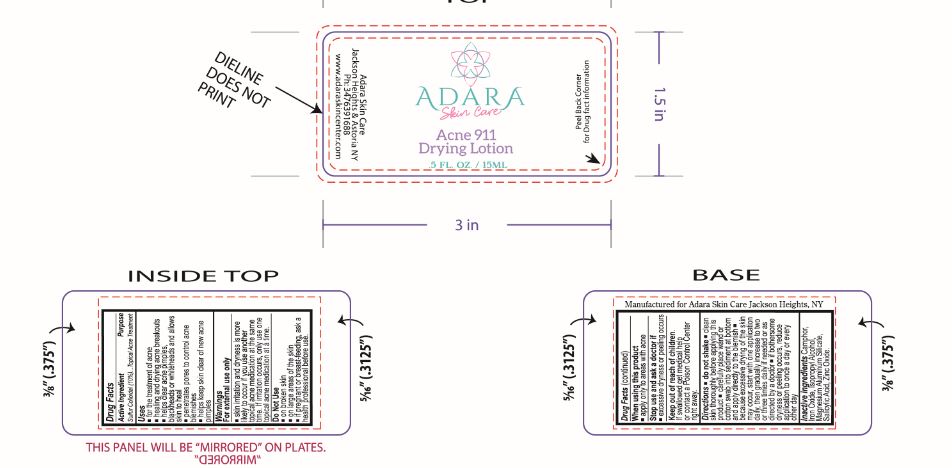
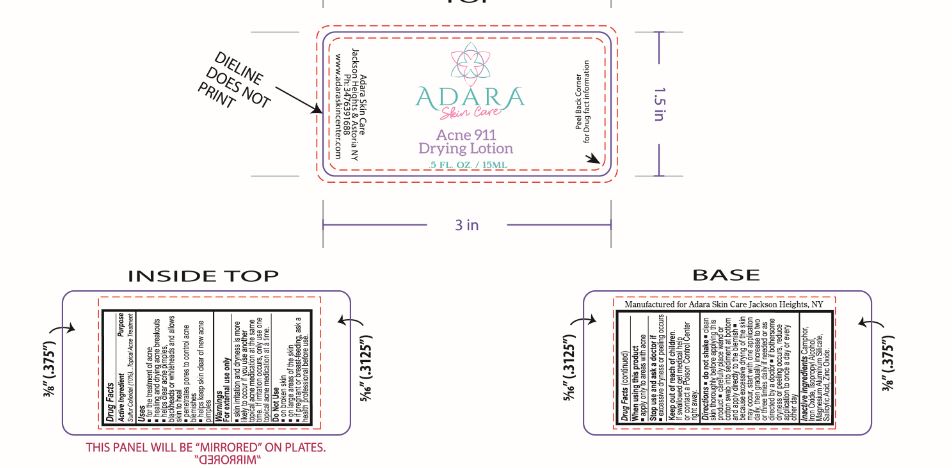
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Do Not Shake
- clean skin thoroughly before applying this product
- carefully place wand or cotton swab into sediment at bottom and apply directly to the blemish
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed
by a doctor - if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ADARA ACNE 911 DRYING
acne drying lotion suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76348-890 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 1.4 g in 14 g Inactive Ingredients Ingredient Name Strength MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) ZINC OXIDE (UNII: SOI2LOH54Z) SALICYLIC ACID (UNII: O414PZ4LPZ) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) ISOPROPYL ALCOHOL (UNII: ND2M416302) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76348-890-01 14 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 08/14/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/14/2023 Labeler - Renu Laboratories, Inc. (945739449) Establishment Name Address ID/FEI Business Operations Renu Laboratories, Inc. 945739449 manufacture(76348-890)