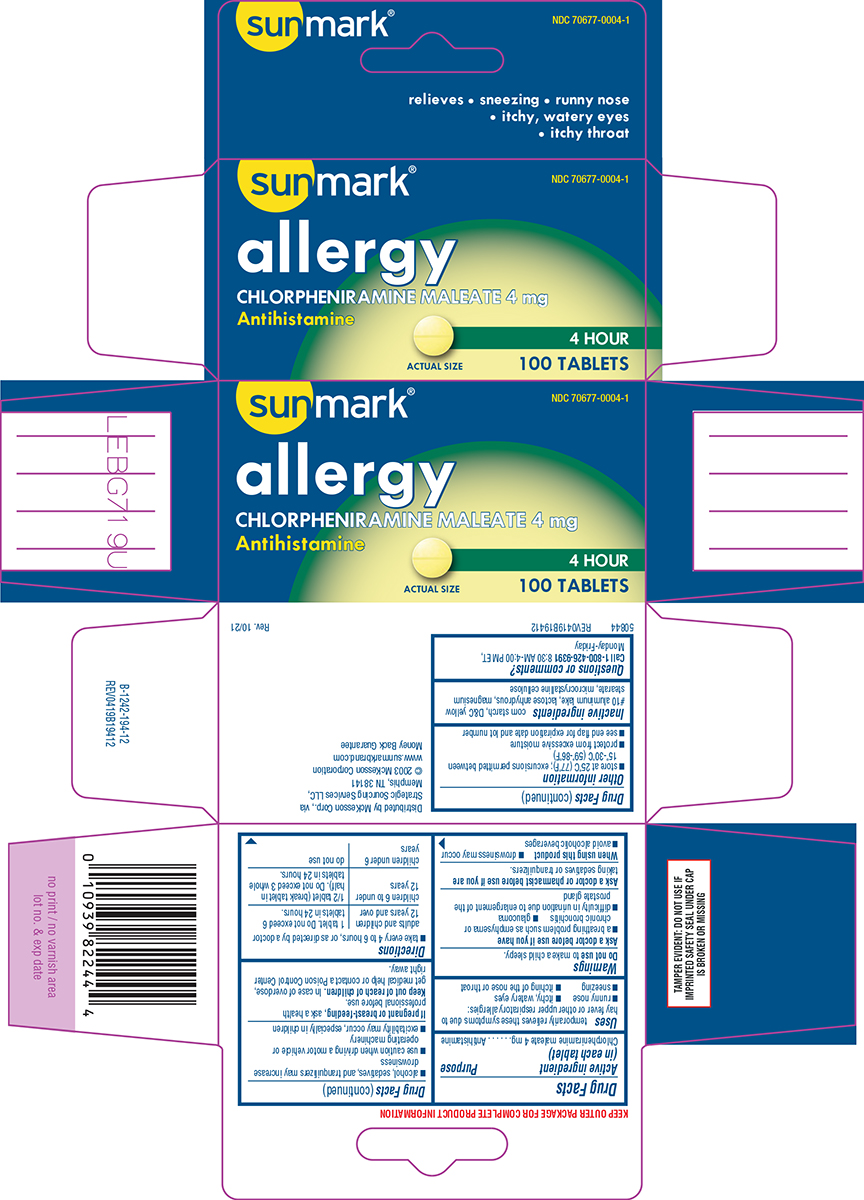
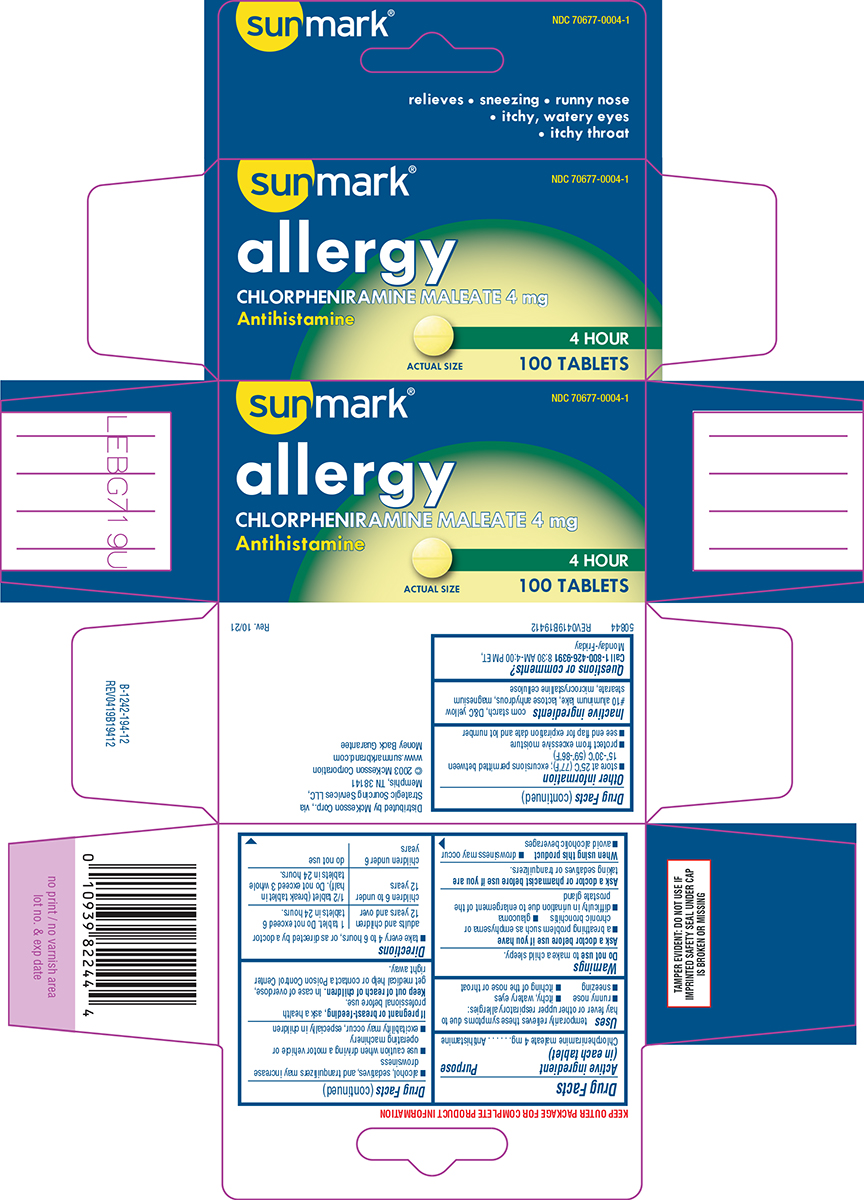
Label: ALLERGY- chlorpheniramine maleate tablet
- NDC Code(s): 70677-0004-1
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
sunmark®
NDC 70677-0004-1
allergy
CHLORPHENIRAMINE MALEATE 4 mg
Antihistamine4 HOUR
100 TABLETS
ACTUAL SIZE
relieves • sneezing • runny nose
• itchy, watery eyes
• itchy throatTAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER CAP
IS BROKEN OR MISSINGDistributed by McKesson Corp., via
Strategic Sourcing Services LLC,
Memphis, TN 38141
© 2003 McKesson Corporation
www.sunmarkbrand.com
Money Back Guarantee50844 REV0419B19412
Sunmark 44-194
-
INGREDIENTS AND APPEARANCE
ALLERGY
chlorpheniramine maleate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70677-0004 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color yellow Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code 44;194 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70677-0004-1 1 in 1 CARTON 12/19/1992 03/16/2025 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/19/1992 03/16/2025 Labeler - Strategic Sourcing Services LLC (116956644) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(70677-0004) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(70677-0004) , pack(70677-0004) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(70677-0004) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(70677-0004) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(70677-0004)