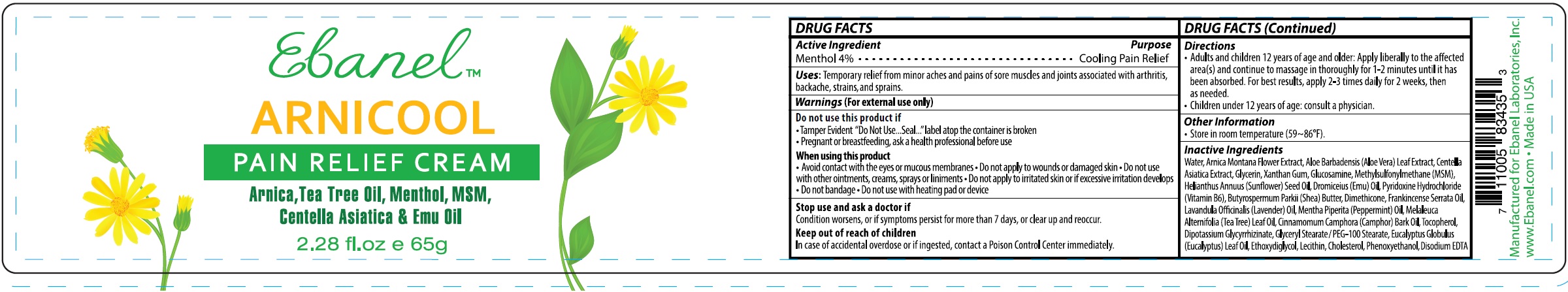
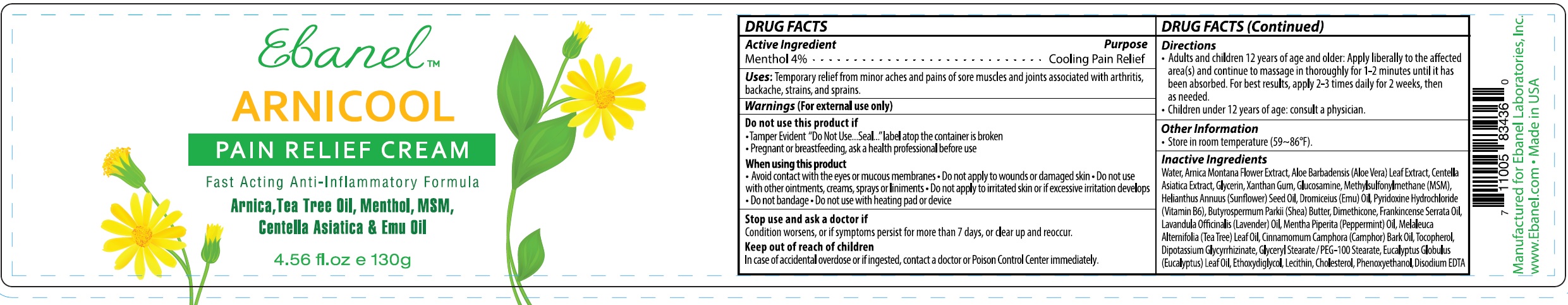
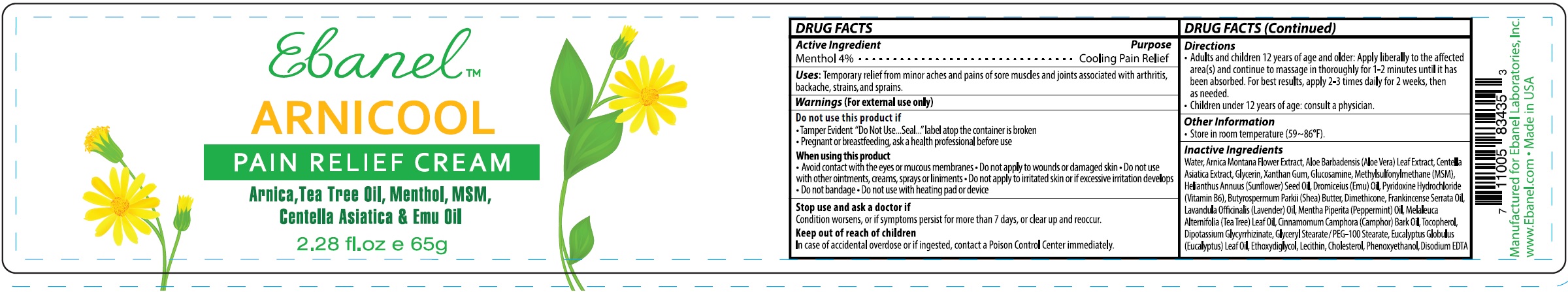
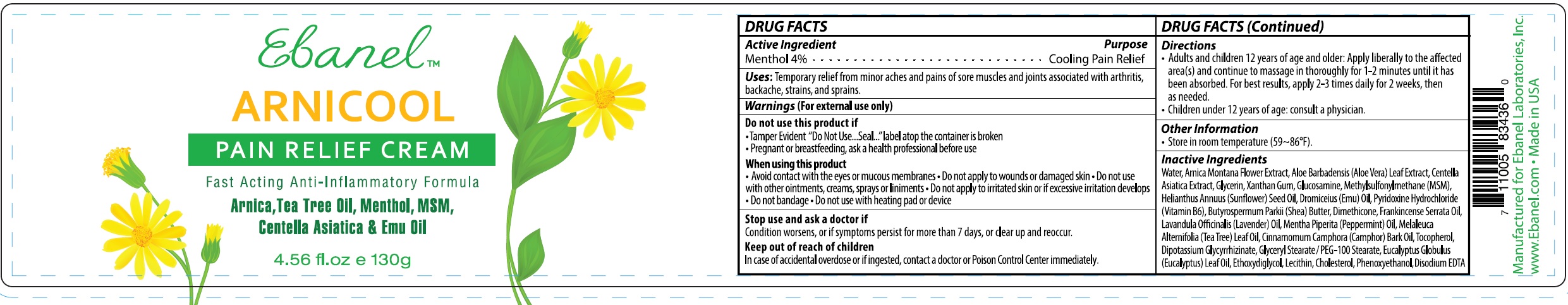
Label: ARNICOOL- menthol cream
- NDC Code(s): 63742-007-00, 63742-007-01
- Packager: Clinical Resolution Laboratory, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- Active Ingredient
- Uses
-
Warnings
(For external use only)
Do not use this product if
- Tamper Evident "Do Not Use...Seal..."label atop the container is broken
- Pregnant or breastfeeding, ask a health professional before use
When using this product
- Avoid contact with the eyes or mucous membranes
- Do not apply to wounds or damaged skin
- Do not use with other ointments, creams, sprays or liniments
- Do not apply to irritated skin or if excessive irritation develops
- Do not bandage
- Do not use with heating pad or device
- Directions
- Other Information
-
Inactive Ingredients
Water, Arnica Montana Flower Extract, Aloe Barbadensis (Aloe Vera) Leaf Extract, Centella Asiatica Extract, Glycerin, Xanthan Gum, Glucosamine, Methylsulfonylmethane (MSM), Helianthus Annuus (Sunflower) Seed Oil, Dromiceius (Emu) Oil, Pyridoxine Hydrochloride (Vitamin B6), Butyrospermum Parkii (Shea) Butter, Dimethicone, Frankincense Serrata Oil, Lavandula Officinalis (Lavender) Oil, Mentha Piperita (Peppermint) Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Cinnamomum Camphora (Camphor) Bark Oil, Tocopherol, Dipotassium Glycyrrhizinate, Glyceryl Stearate / PEG-100 Stearate, Eucalyptus Globulus (Eucalyptus) Leaf Oil, Ethoxydiglycol, Lecithin, Cholesterol, Phenoxyethanol, Disodium EDTA
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ARNICOOL
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63742-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 40 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) ALOE VERA LEAF (UNII: ZY81Z83H0X) CENTELLA ASIATICA (UNII: 7M867G6T1U) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) GLUCOSAMINE (UNII: N08U5BOQ1K) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) EMU (UNII: 97P687I7AP) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) SHEA BUTTER (UNII: K49155WL9Y) DIMETHICONE (UNII: 92RU3N3Y1O) LAVENDER OIL (UNII: ZBP1YXW0H8) PEPPERMINT OIL (UNII: AV092KU4JH) TEA TREE OIL (UNII: VIF565UC2G) CAMPHOR OIL (UNII: 75IZZ8Y727) TOCOPHEROL (UNII: R0ZB2556P8) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) EUCALYPTUS GUM (UNII: 72T9EZC2VX) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) CHOLESTEROL (UNII: 97C5T2UQ7J) PHENOXYETHANOL (UNII: HIE492ZZ3T) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63742-007-00 65 g in 1 JAR; Type 0: Not a Combination Product 05/15/2018 2 NDC:63742-007-01 130 g in 1 JAR; Type 0: Not a Combination Product 05/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/15/2018 Labeler - Clinical Resolution Laboratory, Inc. (825047942)