Label: SIMPLIFY ANTIBACTERIAL HAND WIPES- benzalkonium chloride cloth
- NDC Code(s): 11822-0283-1
- Packager: Rite Aid Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
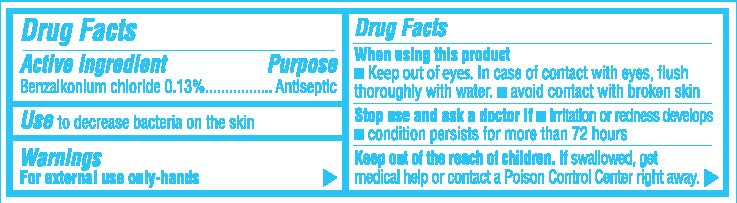
- Active Ingredient
- Purpose
- Use
- Warnings
- SPL UNCLASSIFIED SECTION
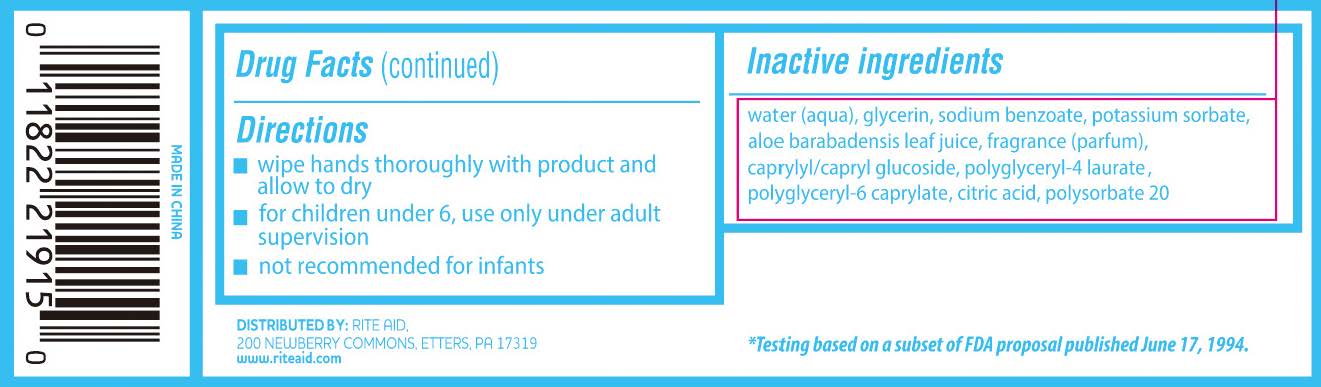
- Directions
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- 20 Wipes 5.5 x 7 in (14 x 17.8 cm)
-
INGREDIENTS AND APPEARANCE
SIMPLIFY ANTIBACTERIAL HAND WIPES
benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-0283 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYGLYCERYL-6 CAPRYLATE (UNII: DGV8R54VG7) SODIUM BENZOATE (UNII: OJ245FE5EU) POLYGLYCERYL-4 LAURATE (UNII: 82V7NG3DYT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-0283-1 20 in 1 POUCH; Type 0: Not a Combination Product 09/11/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/11/2023 Labeler - Rite Aid Corporation (014578892)