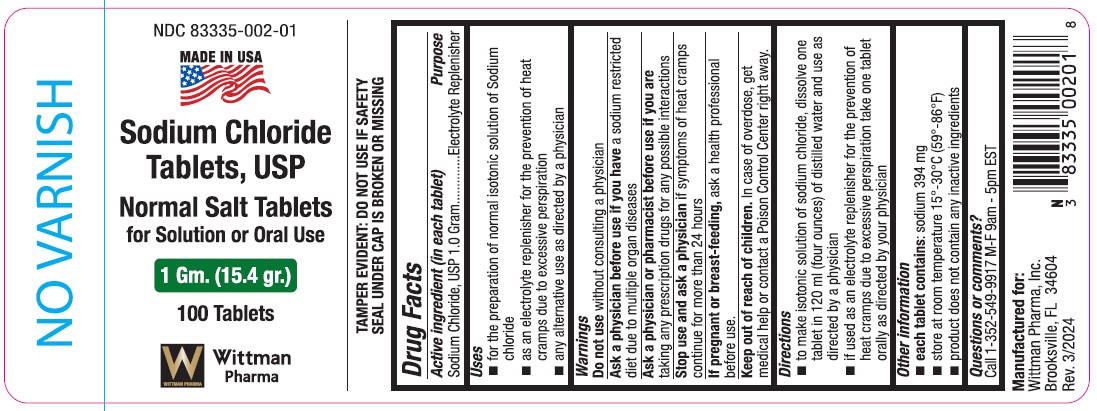
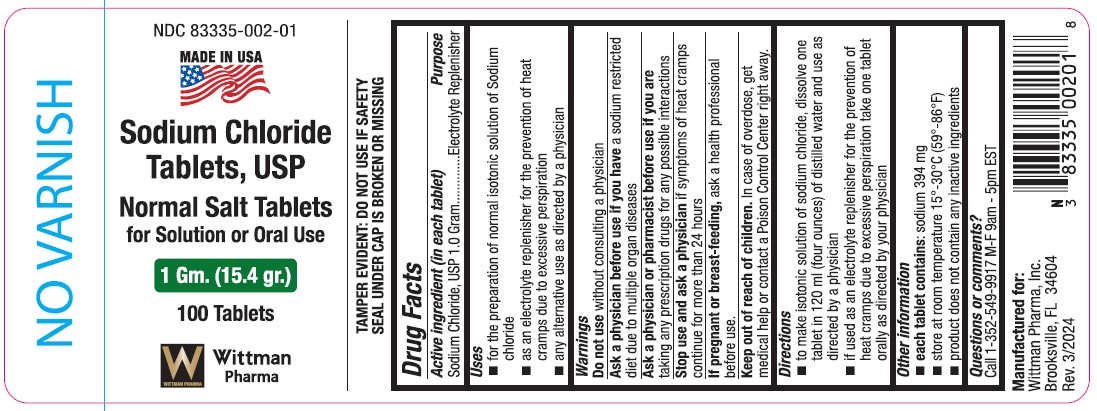
Label: SODIUM CHLORIDE- normal salt tablets tablet
- NDC Code(s): 83335-002-01
- Packager: Wittman Pharma, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DOSAGE & ADMINISTRATION
Directions
- to make isontonic solution of sodium chloride, dissolve one tablet in 120 ml (four ounces) of distilled water and use as directed by a physician.
- if used as an electrolyte replenisher for the prevention of heat cramps due to excessive perspiration take one tablet orally as directed by your physician.
-
WARNINGS
Warnings
Do not use without consulting a physician
Ask a physician before use if you have a sodium restricted diet due to multiple organ diseases
Stop use and ask a physician if symptoms of heat cramps continue for more than 24 hours.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- ACTIVE INGREDIENT
-
Drug Facts
Active ingredient (in each tablet)
Sodium Chloride, USP 1.0 gram
Purpose
Electrolyte Replenisher
Uses
- for the preparation of normal isotonic solution of sodium chloride
- as an electrolyte replenisher for the prevention of heat cramps due to excessive perspiration
- any alternative use as directed by a physician
Warnings
Do not use without consulting a physician
Ask a physician before use if you have a sodium restricted diet due to multiple organ diseases
Stop use and ask a physician if symptoms of heat cramps continue for more than 24 hours.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- to make isontonic solution of sodium chloride, dissolve one tablet in 120 ml (four ounces) of distilled water and use as directed by a physician.
- if used as an electrolyte replenisher for the prevention of heat cramps due to excessive perspiration take one tablet orally as directed by your physician.
Other information
- each tablet contains: sodium 394 mg
- store at room temperature 15°-30°C (59°-86°F)
- product does not contain any inactive ingredients
Questions or comments?
Call 1-352-549-9917 M-F 9am - 5pm EST
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
normal salt tablets tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83335-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 1 g in 1 g Product Characteristics Color white Score no score Shape ROUND Size 12mm Flavor Imprint Code SC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83335-002-01 100 g in 1 BOTTLE; Type 0: Not a Combination Product 08/09/2023 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/09/2023 Labeler - Wittman Pharma, Inc. (830980947) Registrant - Wittman Pharma, Inc. (830980947) Establishment Name Address ID/FEI Business Operations Wittman Pharma, Inc. 830980947 label(83335-002) , analysis(83335-002) , manufacture(83335-002)