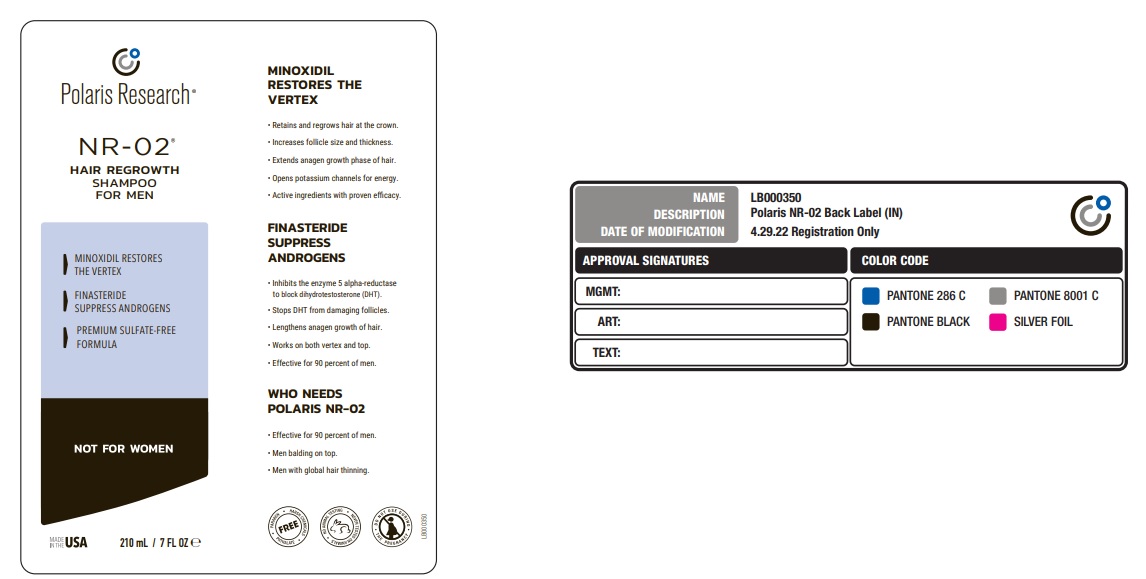
Label: POLARIS RESEARCH NR-02 HAIR REGROWTH- minoxidil shampoo
- NDC Code(s): 69188-400-07
- Packager: DS Healthcare Group
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated February 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- PURPOSE
- Use
-
Warning
For external use only. Avoid eye contact. This product is not for women.Finasteride has been shown to cause birth defects in women who are pregnant or may become pregnant. Be sure this product is never handled or used by women.
Keep out of reach of children.
Minoxidil has been shown to increase unwanted body hair. Use as directed. - KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
-
Inactive ingredients
WATER, DISODIUM LAURETH SULFOSUCCINATE, SODIUM COCOYL ISETHIONATE, COCAMIDOPROPYL HYDROXYSULTAINE, SODIUM LAUROYL
SARCOSINATE, AMODIMETHICONE, C11-15 PARETH-7, GLYCERIN, LAURETH-9, PEG-150 DISTEARATE, TRIDECETH-12, COCAMIDE MEA,
DI-PPG2 MYRETH-10 ADIPATE, SODIUM LAURYL SULFOACETATE, COCAMIDOPROPYLAMINE OXIDE, GLYCOL DISTEARATE, MINOXIDIL,
CAFFEINE, POLYSORBATE 20, KETOCONAZOLE, FINASTERIDE, BIOTIN, COPPER TRIPEPTIDE-1,TOCOPHEROL, RETINYL PALMITATE, AESCULUS
HIPPOCASTANUM (HORSE CHESTNUT) SEED EXTRACT, ASPALATHUS LINEARIS LEAF EXTRACT, HYDROLYZED SOY PROTEIN,HYDROLYZED WHEAT PROTEIN, WHEAT AMINO ACIDS, INOSITOL, PHENOXYETHANOL,CINNAMIDOPROPYLTRIMONIUM CHLORIDE,HYDROLYZED PROANTHOCYANIDIN, CAPRYLYL GLYCOL, METHYL SULFONYL METHANE,ALCOHOL DENAT, CALCIUM PANTOTHENATE,LINOLEIC ACID, PEG-35 CASTOR OIL,
POLYQUATERNIUM 10 , TAURINE, TETRASODIUM EDTA, ETHYLBISIMINOMETHYLGUAIACOLMANGANESE CHLORIDE, FRAGRANCE CITRIC ACID - Product label
-
INGREDIENTS AND APPEARANCE
POLARIS RESEARCH NR-02 HAIR REGROWTH
minoxidil shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69188-400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 1.5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) COCAMIDOPROPYL HYDROXYSULTAINE (UNII: 62V75NI93W) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) AMODIMETHICONE (800 CST) (UNII: 363Z2T48P7) C11-15 PARETH-7 (UNII: 261HPE0IS3) GLYCERIN (UNII: PDC6A3C0OX) POLIDOCANOL (UNII: 0AWH8BFG9A) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) TRIDECETH-12 (UNII: YFY3KG5Y7O) COCO MONOETHANOLAMIDE (UNII: C80684146D) DI-PPG-2 MYRETH-10 ADIPATE (UNII: 4IN301M0KJ) SODIUM LAURYL SULFOACETATE (UNII: D0Y70F2B9J) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) GLYCOL DISTEARATE (UNII: 13W7MDN21W) CAFFEINE (UNII: 3G6A5W338E) POLYSORBATE 20 (UNII: 7T1F30V5YH) KETOCONAZOLE (UNII: R9400W927I) FINASTERIDE (UNII: 57GNO57U7G) BIOTIN (UNII: 6SO6U10H04) PREZATIDE COPPER (UNII: 6BJQ43T1I9) TOCOPHEROL (UNII: R0ZB2556P8) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) HORSE CHESTNUT (UNII: 3C18L6RJAZ) ASPALATHUS LINEARIS LEAF (UNII: H7UGK1GJCU) SOY PROTEIN (UNII: R44IWB3RN5) HYDROLYZED WHEAT PROTEIN (ENZYMATIC; 3000 MW) (UNII: J2S07SB0YL) AMINO ACIDS, WHEAT (UNII: 0370GZL32F) INOSITOL (UNII: 4L6452S749) PHENOXYETHANOL (UNII: HIE492ZZ3T) ACETYLCYSTEINE SODIUM (UNII: NRD80R06FB) PROCYANIDIN A2 (UNII: UQN6668Q4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) ALCOHOL (UNII: 3K9958V90M) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) LINOLEIC ACID (UNII: 9KJL21T0QJ) POLYOXYL 35 CASTOR OIL (UNII: 6D4M1DAL6O) POLYQUATERNIUM-10 (20000 MPA.S AT 2%) (UNII: N2GK4S7X4T) TAURINE (UNII: 1EQV5MLY3D) EDETATE SODIUM (UNII: MP1J8420LU) ETHYLBISIMINOMETHYLGUAIACOL MANGANESE CHLORIDE (UNII: SM5YJ88LTU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69188-400-07 210 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/22/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/22/2015 Labeler - DS Healthcare Group (015504134) Establishment Name Address ID/FEI Business Operations A.I.G. TECHNOLOGIES, INC. 086365223 manufacture(69188-400)