Label: MECLIZINE HCL 25 MG- meclizine hcl tablet
-
NDC Code(s):
71335-1607-0,
71335-1607-1,
71335-1607-2,
71335-1607-3, view more71335-1607-4, 71335-1607-5, 71335-1607-6, 71335-1607-7, 71335-1607-8, 71335-1607-9
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 69618-028
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
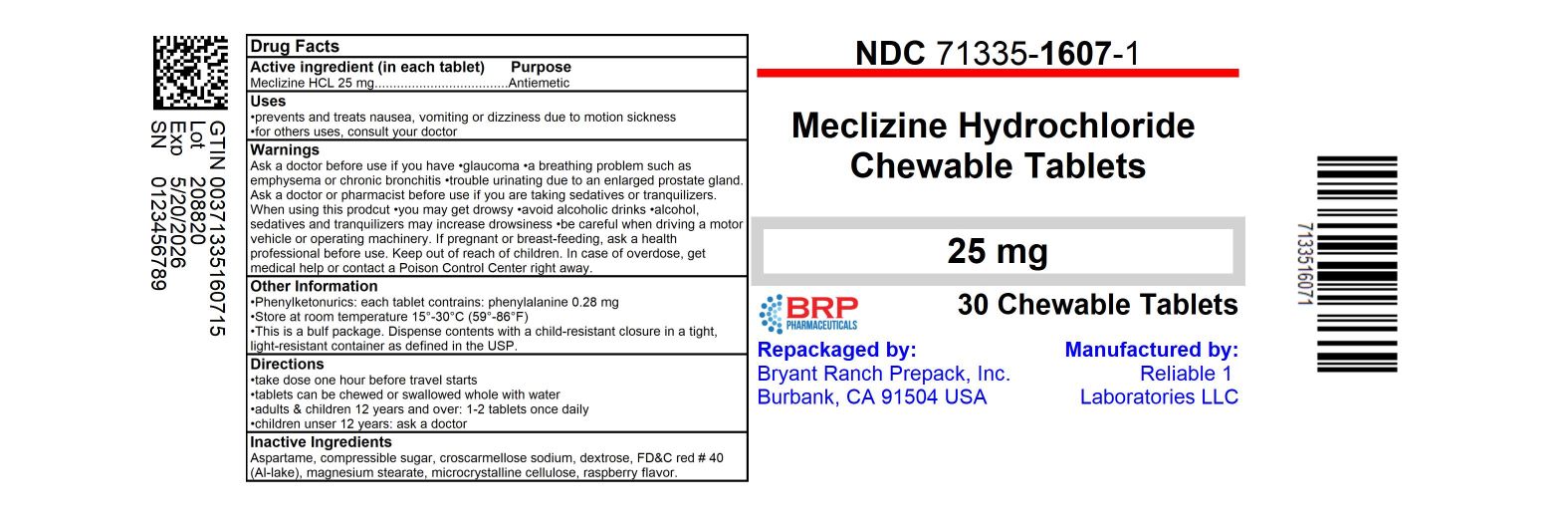
- Drug Facts
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this prodcut
- you may get drowsy
- avoid alcoholic drinks
- alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
HOW SUPPLIED
NDC: 71335-1607-1: 30 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1607-2: 20 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1607-3: 25 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1607-4: 40 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1607-5: 60 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1607-6: 90 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1607-7: 8 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1607-8: 14 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1607-9: 10 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1607-0: 120 Tablets in a BOTTLE, PLASTIC
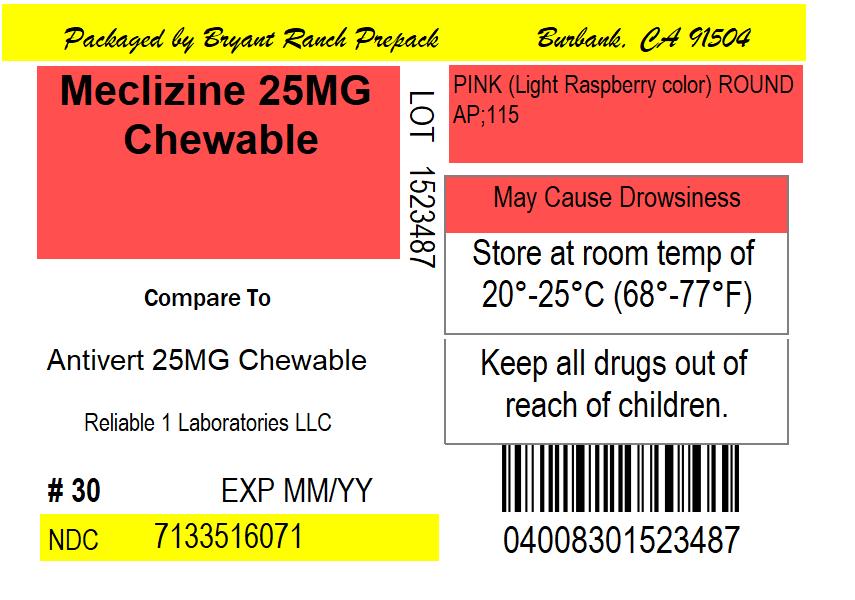
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MECLIZINE HCL 25 MG
meclizine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71335-1607(NDC:69618-028) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) RASPBERRY (UNII: 4N14V5R27W) ASPARTAME (UNII: Z0H242BBR1) SUCROSE (UNII: C151H8M554) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) Product Characteristics Color pink (Light Raspberry color) Score 2 pieces Shape ROUND Size 8mm Flavor RASPBERRY Imprint Code AP;115 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71335-1607-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/29/2020 2 NDC:71335-1607-2 20 in 1 BOTTLE; Type 0: Not a Combination Product 06/15/2020 3 NDC:71335-1607-3 25 in 1 BOTTLE; Type 0: Not a Combination Product 09/10/2021 4 NDC:71335-1607-4 40 in 1 BOTTLE; Type 0: Not a Combination Product 02/14/2022 5 NDC:71335-1607-5 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/03/2022 6 NDC:71335-1607-6 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/24/2020 7 NDC:71335-1607-7 8 in 1 BOTTLE; Type 0: Not a Combination Product 02/14/2022 8 NDC:71335-1607-8 14 in 1 BOTTLE; Type 0: Not a Combination Product 02/14/2022 9 NDC:71335-1607-9 10 in 1 BOTTLE; Type 0: Not a Combination Product 09/09/2021 10 NDC:71335-1607-0 120 in 1 BOTTLE; Type 0: Not a Combination Product 02/14/2022 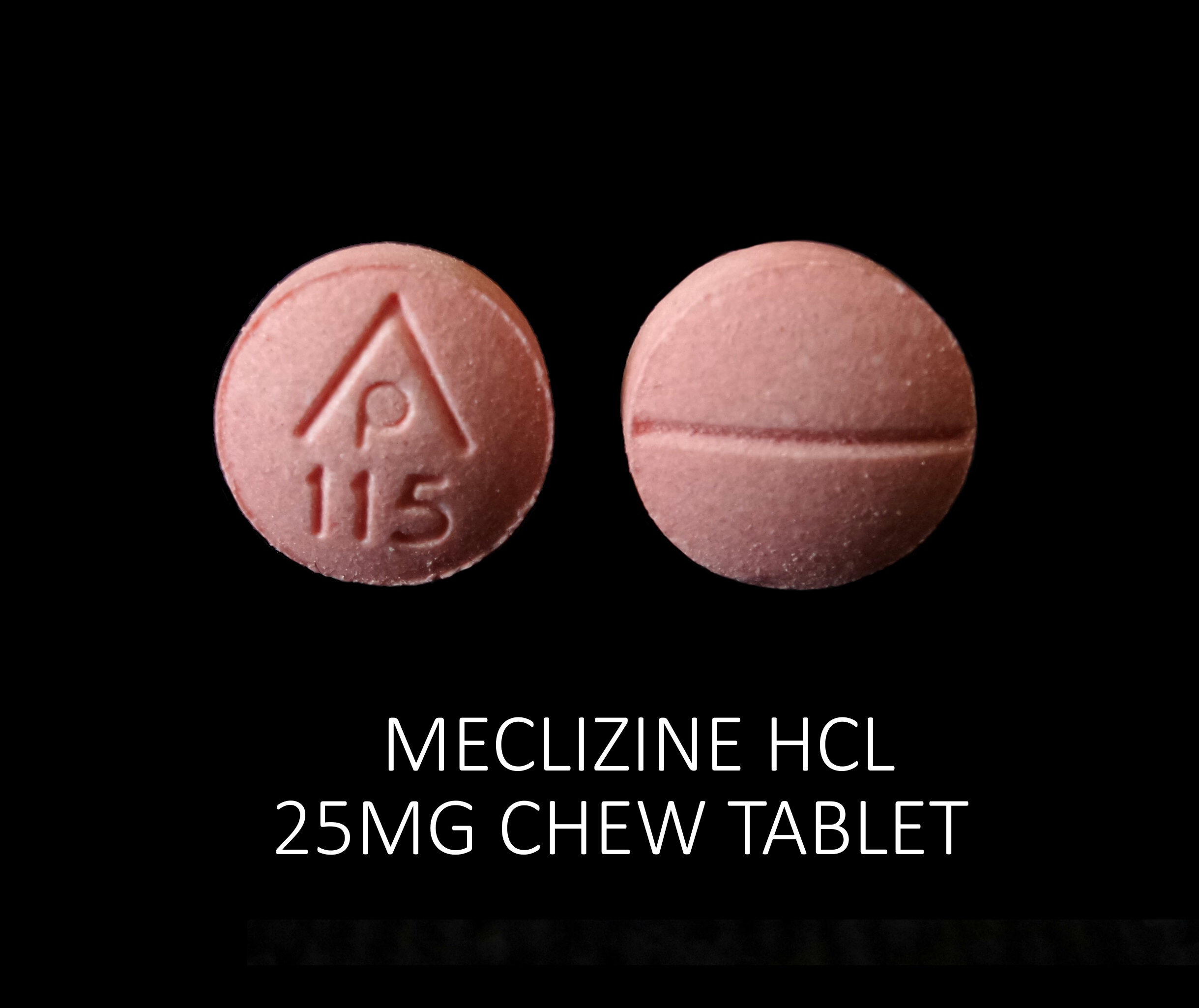
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug part336 11/01/2015 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(71335-1607) , RELABEL(71335-1607)