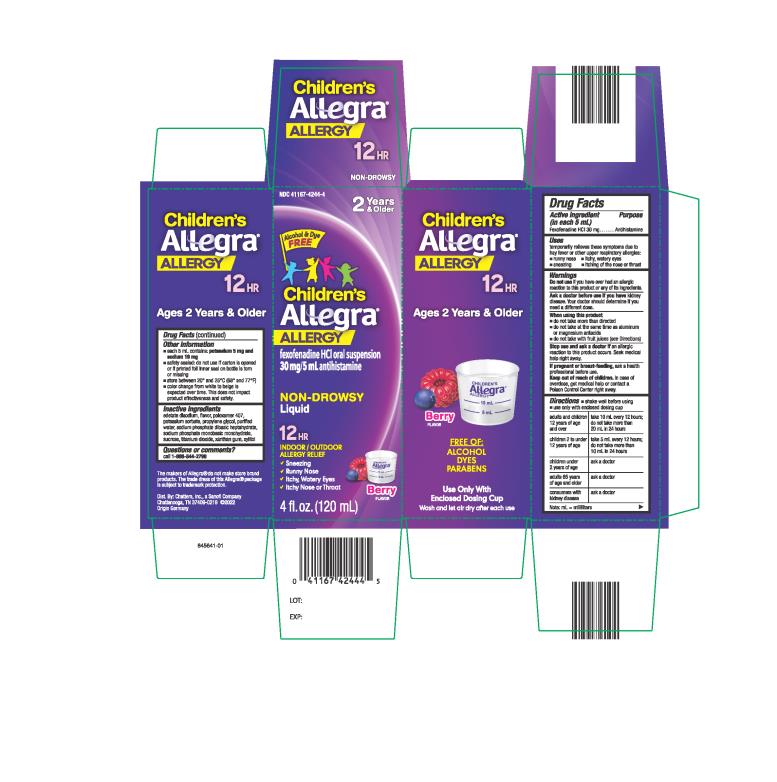
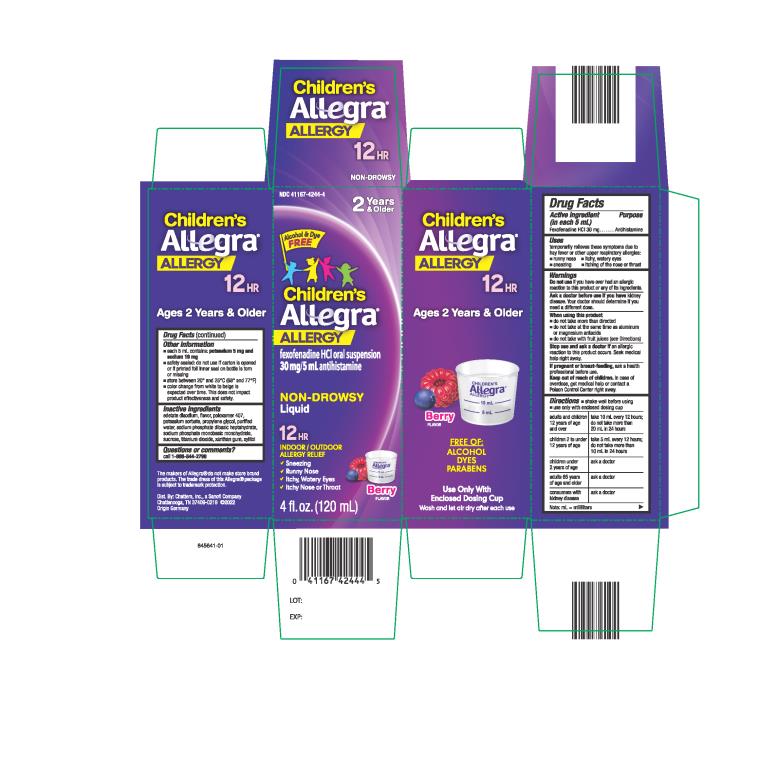
Label: CHILDRENS ALLEGRA ALLERGY- fexofenadine hydrochloride suspension
-
NDC Code(s):
41167-4244-1,
41167-4244-4,
41167-4244-7,
41167-4246-2, view more41167-4246-4
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- do not take more than directed
-
Directions
- shake well before using
- use only with enclosed dosing cup
adults and children 12 years of age and over take 10 mL every 12 hours; do not take more than 20 mL in 24 hours children 2 to under 12 years of age take 5 mL every 12 hours; do not take more than 10 mL in 24 hours children under 2 years of age ask a doctor adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor Note: mL = milliliters
- shake well before using
-
Other information
- each 5 mL contains: each 5 mL contains: potassium 5 mg and sodium 19 mg
- safety sealed: do not use if carton is opened or if printed foil inner seal on bottle is torn or missing
- store between 20° and 25°C (68° and 77°F)
- color change from white to beige is expected over time. This does not impact product effectiveness and safety.
- each 5 mL contains: each 5 mL contains: potassium 5 mg and sodium 19 mg
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLEGRA ALLERGY
fexofenadine hydrochloride suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-4244 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) POLOXAMER 407 (UNII: TUF2IVW3M2) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color white Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-4244-4 1 in 1 CARTON 03/03/2011 1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:41167-4244-1 1 in 1 CARTON 03/03/2011 2 240 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:41167-4244-7 3 in 1 CELLO PACK 03/03/2011 3 120 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA201373 03/03/2011 CHILDRENS ALLEGRA ALLERGY
fexofenadine hydrochloride suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-4246 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) POLOXAMER 407 (UNII: TUF2IVW3M2) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color white Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-4246-4 1 in 1 CARTON 03/01/2023 1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:41167-4246-2 1 in 1 CARTON 03/01/2023 2 240 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA201373 03/03/2011 Labeler - Chattem, Inc. (003336013)