Label: PRIBUTAZONE- phenylbutazone tablet
- NDC Code(s): 58829-334-10
- Packager: FIRST PRIORITY INCORPORATED
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated November 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
-
CAUTION:
Federal law restricts this drug to use by closed when not in use. or on the order of a licensed veterinarian. Federal law prohibits the extra-label use of this product in female dairy cattle 20 months of age or older.
Not for Use in Humans • Keep Out of Reach of Children
Approved by FDA under ANADA # 200-433
-
DESCRIPTION & PHARMACOLOGY:
Phenylbutazone chemically is 4-butyl-1, 2- diphenyl-3, 5-pyrazolidinedione. It has the following structural formula:
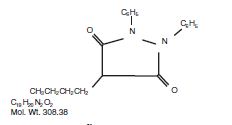
Phenylbutazone was first synthesized in 1948 and introduced into human medicine in 1949, Kuzell1,2,3, Payne4, Fleming5, and Denko6, demonstrated the clinical effectiveness of phenylbutazone in gout, gouty arthritis, acute arthritis, acute rheumatism and various other rheumatoid disorders in humans. Fabre7, Domenjoz8, Wilhelmi9, and Yourish10, have established the anti-rheumatic and anti-inflammatory activity of phenylbutazone. It is entirely unrelated to the steroid hormones.
Toxicity of phenylbutazone has been investigated in rats and mice11, Ogilvie and Sutter12, have also made a study on the chronic toxicity of phenylbutazone in dogs. They have shown that dogs receiving 10 mg. and 100 mg. per Kg. body weight, per day for 90 days, maintain good appetites, excrete normal feces, gain weight and maintain a normal blood picture. They also report no abnormal macroscopic or microscopic changes in sacrificed animals which could have been attributed to the drug.
Phenylbutazone has been used by Camberos13 in thoroughbred horses. Favorable results were reported in cases of traumatism, muscle rupture, strains and inflammations of the third phalanx. Results were not as favorable in the periodic treatment of osteo-arthritis of medial and distal bones of the hock, arthritis of the stifle and hip, arthrosis of the trapezious muscles, and generalized arthritis. Sutter14 reported a favorable response in chronic equine arthritis of long duration, fair results in a severely bruised mare, and poor results in two cases where the condition was limited to the third phalanx. - INDICATIONS:
- DOSAGE & ADMINISTRATION:
- INGREDIENTS:
- CONTRAINDICATIONS:
- PRECAUTION:
- WARNING:
-
CONTACT INFORMATION:
To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact First Priority, Inc. at (800) 650-4899 or www.prioritycare.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae
- CAUTION:
- AVAILABILITY:
- STORAGE:
-
REFERENCES
1. Kuzell, W.C., Schafferzick, R.W., Naughler, W.E., Gandia, G. and Mankle, E.A.: A.M.A. Arch. Inst. Med., 92; 646 (1953).
2. Kuzell, W.C., Schafferzick, R.W., Brown, B. and Mankle, E.A.: J.A.M.A. 149; 729 (1952).
3. Kuzell, W.C., and Schafferzick, R.W.: Calif. Med. 77; 319 (1952).
4. Payne, R.W., Shelter, M.R., Farr, C.H., Hellbaum, A.A. and Ishmall, W.K.: J. Lab. Clin. Med., 45; 331 (1955).
5. Fleming, J., and Will, G.: Ann. Rheumat. Dist., 12; 95 (1953).
6. Denko, C.W., and Rumi, D.: American Pract. 6; 1865 (1956).
7. Fabre, J., et al: Semain. Hop. (Paris) 31; 87 (1955).
8. Domenjoz, R., et al: Arzneimittel-Forsch, 5; 488 (1955).
9. Wilhelmi, G. and Pulver, R.: Arzneimittel- Forsch, 5; 221 (1955).
10. Yourish, W., Paton, B., Brodie, B., Burns, J.: A.M.A. Arch. Ophth., 53; 264 (1955).
11. Hazelton, L.W., Tusing, T.W. and Hollana, E.G.: J. Pharmacol. Exper. Ther., 109; 387 (1953).
12. Ogilvie, F.B. and Sutter, M.D.: Vet. Med. 52; 492-4 (1957).
13. Camberos, H.R.: Rev. Med. Vet. (Buenos Aires) 38; 9 (1956).
14. Sutter, M.D.: Vet. Med., 53; 83 (Feb. 1958). - INFORMATION FOR OWNERS/CAREGIVERS
- Net Contents: 100 Boluses
-
INGREDIENTS AND APPEARANCE
PRIBUTAZONE
phenylbutazone tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:58829-334 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLBUTAZONE (UNII: GN5P7K3T8S) (PHENYLBUTAZONE - UNII:GN5P7K3T8S) PHENYLBUTAZONE 1 g Product Characteristics Color white (WHITE) Score 2 pieces Shape ROUND (ROUND) Size 18mm Flavor Imprint Code BUTE;1GM Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58829-334-10 12 in 1 CASE 1 100 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200433 09/21/2023 Labeler - FIRST PRIORITY INCORPORATED (179925722) Establishment Name Address ID/FEI Business Operations FIRST PRIORITY INCORPORATED 179925722 manufacture



